Tags | |
UUID | 19af3dc2-f145-11e9-8682-bc764e2038f2 |
Chapter 12 Problems
1. When 1 mol of fuel is burned at constant pressure, it produces 3452 kJ of heat and does 11 kJ of work. What is the value of ΔU for the combustion of the fuel?
2. Consider the following thermal chemical equation for the combustion of acetone (C_3H_6O), the main ingredient in nail polish remover:
C3H6O(l)+4O2(g)→3CO2(g)+3H2O(g) ΔHrxn=-1790kJ
If a bottle of nail polish remover contains 177mL of acetone, how much heat would be released by its complete combustion? The density of acetone is 0.788 g/mL.
3. A calorimeter contained 75.0 g of water at 16.95°C. A 93.3 g iron sample at 65.58°C was placed in it, giving a final temperature of 19.68°C for the system. Calculate the heat capacity of the calorimeter. Specific heats are 4.184 J/g·°C for water and 0.444 J/g·°C for iron.
4.Calculate the standard enthalpy of combustion of the transition of C(s, graphite) → C(s, diamond), given
C(s, graphite) + O2 → CO2 ΔH0 =-393.5kJ/mol
CO2 → C(s,⋄)+O2 ΔH0 =+395.41kJ/mol
5. 1.150 g of sucrose goes through combustion in a bomb calorimeter. If the temperature rose from 23.42°C to 27.64°C and the heat capacity of the calorimeter is 4.90 kJ/°C, then determine the heat of combustion of sucrose, C12H22O11, in kj per mole of C12H22O12.
6. Without doing a calculation, predict whether the entropy change will be positive or negative.
a) C2H6(g)+72O2(g)→3H2O(g)+2CO2(g)
b) 3C2H2(g)→C6H6(l)
c) C^H12O6(s)+6O2(g)→6CO2(g)+6H2O(l)
7. Calculate the standard-state free energy of formation for the H2O2(l) from H2 and O2, given the following values:
ΔGf (H2): 0 kJ/mol
ΔGf (O2): 0 kJ/mol
ΔGf (H2O2(l)): -120.4 kJ/mol
8.
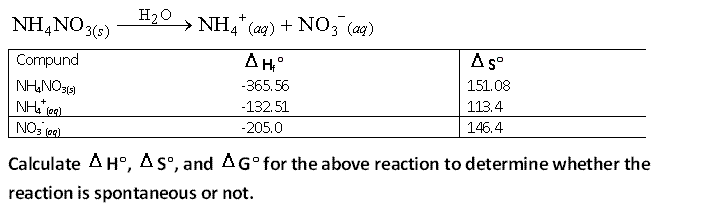
9.

10. The change in internal energy for the combustion of 1.0 mol of octane at a pressure of 1.0 atm is 5084.3 kJ. If the change in heat energy is 5074.1 kJ, how much work is done during the combustion?
11. The propane fuel (C3H8) used in gas barbeques burns according to the following thermochemical equation: C3H8(g)+5O2(g)→3CO2 (g)+4H2O(g) ΔHrxn=-2217kJ
If a pork roast must absorb 160 kJ to fully cook, and if only 10% of the heat produced by the BBQ is actually absorbed by the roast, what mass of CO2 is emitted into the atmosphere during the grilling of the porkroast?
12. A 5.1g piece of gold jewelry is removed from water at 100.0°C and placed in a coffee-cup calorimeter containing 16.9 g of water at 22.5°C. The equilibrium temperature is 23.2°C. The calorimeter constant is known to be 1.54 J/°C. What is the specific heat of this piece of jewelry? The specific heat of pure gold is 0.129J/g°C. Is the jewelery pure gold?
13. Use the tabulated bond energies to estimate the enthalpy of the following reaction: CO+H2O→CO2 +H2
14. Given the following data calculate the heat of combustion in kJ/mol of xylose, C5H10O5(s), used in a bomb calorimetry experiment: mass of C5H10O5(s)=1.250g, heat capacity of calorimeter = 4.728 kJ/°C, Initial Temperature of the calorimeter = 24.37°C, Final Temperature of calorimeter = 28.29°C.
15. In the following changes is there an increase in entropy?
a) The freezing of water
b) l2(s)→l2(g)
c) The sublimation of dry ice, solid CO2
d) PCl5 (g)→PCl3(g)+Cl2(g)
16. Using the standard molar entropies, calculate the value of ΔS for the following reaction:
4HCl(g)+O2(g)→2Cl2(g)+2H2O(g) .
17. Consider the following reaction:
CaCO3 → CaO+CO2
At what temperature will this reaction become spontaneous? Note: Assume ΔH°r and ΔS°r are temperature independent. (Hint: What variable tells us for sure whether the reaction is spontaneous or not?)
Given values:
| Substance | ΔH°f | S°f |
| CaCO3 | -1206.9 kJ/mol | 92.9 J/K mol |
| CaO | -635.6 kJ/mol | 39.8 J/K mol |
| CO2 | -393.5 kJ/mol | 213.6 J/K mol |
Subpages (1): Chapter 12 Answers to Problems
This Collection is empty
- Comments
- Attachments
- Stats
No comments |
