Tags | |
UUID | 19b54ec1-f145-11e9-8682-bc764e2038f2 |
From UCDavis Chemwiki
Temperature Dependence of Free Energy
Recalling the condition for spontaneous change
ΔG=ΔH–T ΔS<0
it is apparent that the temperature dependence of ΔG depends almost entirely on the entropy change associated with the process. (We say "almost" because the values of ΔH and ΔS are themselves slightly temperature dependent; both gradually increase with temperature). In particular, notice that in the above equation the sign of the entropy change determines whether the reaction becomes more or less spontaneous as the temperature is raised.
For any given reaction, the sign of ΔH can also be positive or negative. This means that there are four possibilities for the influence that temperature can have on the spontaneity of a process:
Case 1: ΔH<0 and ΔS>0
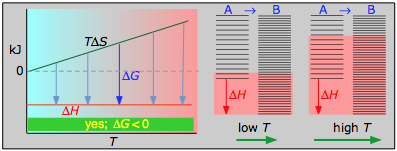
Under these conditions, both the ΔH and TΔS terms will be negative, so ΔG will be negative regardless of the temperature. An exothermic reaction whose entropy increases will be spontaneous at all temperatures.
Case 2: ΔH<0 and ΔS<0
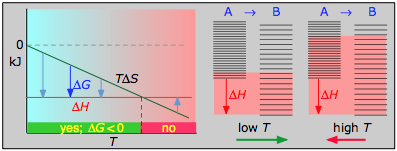
If the reaction is sufficiently exothermic it can force ΔG negative only at temperatures below which |TΔS| <|ΔH|. This means that there is a temperature T=ΔH/ΔS at which the reaction is at equilibrium; the reaction will only proceed spontaneously below this temperature. The freezing of a liquid or the condensation of a gas are the most common examples of this condition.
Case 3: ΔH>0 and ΔS>0
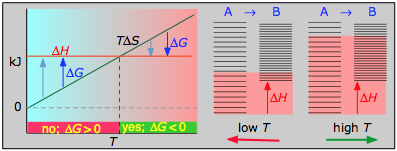
This is the reverse of the previous case; the entropy increase must overcome the handicap of an endothermic process so that TΔS>ΔH. Since the effect of the temperature is to "magnify" the influence of a positive ΔS, the process will be spontaneous at temperatures above T=ΔH/ΔS. (Think of melting and boiling.)
Case 4: ΔH>0 and ΔS<0
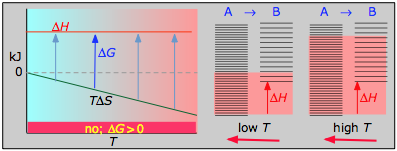
With both ΔH and ΔS working against it, this kind of process will not proceed spontaneously at any temperature. Substance A always has a greater number of accessible energy states, and is therefore always the preferred form.
About the above diagrams
The plots on the left are the important ones; don't try to memorize them, but make sure you understand and can explain or reproduce them for a given set of ΔH and ΔS.
- Their most important differentiating features are the position of the ΔH line (above or below the is TΔS line), and the slope of the latter, which of course depends on the sign of ΔS.
- The reaction A→ B will occur spontaneously only when ΔG is negative (blue arrows pointing down.)
- Owing to the slight temperature dependence of ΔS, the TΔS plots are not quite straight lines as shown here. Similarly, the lines representing ΔH are even more curved.
The other two plots on each diagram are only for the chemistry-committed.
- Each pair of energy-level diagrams depicts the relative spacing of the microscopic energy levels in the reactants and products as reflected by the value of ΔS°. (The greater the entropy, the more closely-spaced are the quantized microstates.)
- The red shading indicates the range of energy levels that are accessible to the system at each temperature. The spontaneous direction of the reaction will always be in the direction in which the red shading overlaps the greater number of energy levels, resulting in the maximum dispersal of thermal energy.
- Note that the vertical offsets correspond to ΔH° for the reaction.
- Never forget that it is the ability of thermal energy to spread into as many of these states as possible that determines the tendency of the process to take place. None of this is to scale, of course!
| The Effect of ΔH, ΔS, and T on Spontaneity | |||
| ΔH | ΔS | Low Temperature | High Temperature |
| - | + | Spontaneous (ΔG<0) | Spontaneous (ΔG<0) |
| + | - | Nonspontaneous (ΔG>0) | Nonspontaneous (ΔG>0) |
| - | - | Spontaneous (ΔG<0) | Nonspontaneous (ΔG>0) |
| + | + | Nonspontaneous (ΔG>0) | Spontaneous (ΔG<0) |
This Collection is empty
- Comments
- Attachments
- Stats
No comments |
