Tags | |
UUID | 19e2512a-f145-11e9-8682-bc764e2038f2 |
Changes in Temperature
From UCDavis Chemwiki
For this, you need to know whether heat is released or absorbed during the reaction. Assume that our forward reaction is exothermic (heat is evolved):
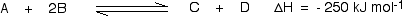
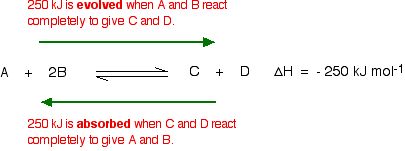
This shows that 250 kJ is evolved (hence the negative sign) when 1 mole of A reacts completely with 2 moles of B. For reversible reactions, the value is always given as if the reaction was one-way in the forward direction. The back reaction (the conversion of C and D into A and B) would be endothermic by exactly the same amount.
The main effect of temperature on equilibrium is in changing the value of the equilibrium constant.
Warning: It is not uncommon that textbooks and instructors to consider temperature as a independent "species" in a reaction. While this is incorrect since once cannot "add or remove temperature" to a reaction as with species, it serves as a convenient mechanism to predict the shift of reactions with changing temperature. For example, if temperature is a "reactant" (ΔH>0), then the reaction favors the formation of products at elevated temperature. Similarly, if temperature is a "product" (ΔH>0), then the reaction favors the formation of reactants. A more accurate, and hence preferred, description is discussed below.
Increasing the temperature
According to Le Châtelier, the position of equilibrium will move in such a way as to counteract the change. That means that the position of equilibrium will move so that the temperature is reduced again. Suppose the system is in equilibrium at 300°C, and you increase the temperature to 500°C. How can the reaction counteract the change you have made? How can it cool itself down again?
To cool down, it needs to absorb the extra heat that you have just put in. In the case we are looking at, the back reaction absorbs heat. The position of equilibrium therefore moves to the left. The new equilibrium mixture contains more A and B, and less C and D.
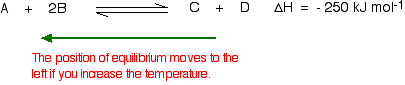
If you were aiming to make as much C and D as possible, increasing the temperature on a reversible reaction where the forward reaction is exothermic isn't a good idea!
Decreasing the temperature?
The equilibrium will move in such a way that the temperature increases again. Suppose the system is in equilibrium at 500°C and you reduce the temperature to 400°C. The reaction will tend to heat itself up again to return to the original temperature. It can do that by favouring the exothermic reaction. The position of equilibrium will move to the right with more A and B are converted into C and D at the lower temperature.

O2 +2H2 ⇌ 2H2O ΔH=-125.7kJ
1. What side of the reaction is favored as you increase the temperature?
2. Would the conversion of O2 and H2O to H2O be favored with heat as a product or as a reactant?
1 Answer: Because the heat is a product of the reaction, the reactants are favored.
2 Answer: Heat as a product would shift the reaction forward, creating H2O. The more heat added to the reaction, the more H2O created
Summary
- Increasing the temperature of a system in dynamic equilibrium favors the endothermic reaction. The system counteracts the change you have made by absorbing the extra heat.
- Decreasing the temperature of a system in dynamic equilibrium favors the exothermic reaction. The system counteracts the change you have made by producing more heat.
Again, this is not in any way an explanation of why the position of equilibrium moves in the ways described. It is only a way of helping you to work out what happens.
Subpages (1): Example 5
This Collection is empty
- Comments
- Attachments
- Stats
No comments |
