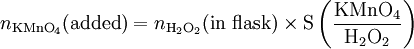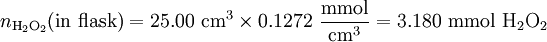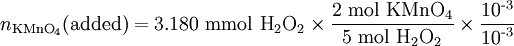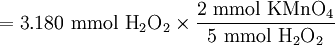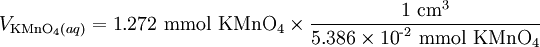Tags | |
UUID | 1a01a54b-f145-11e9-8682-bc764e2038f2 |
Example 3
|
Solution At the equivalence point, the stoichiometric ratio will apply, and we can use it to calculate the amount of KMnO4 which must be added:
|
Calculators and Collections
- Comments
- Attachments
- Stats
No comments |
This site uses cookies to give you the best, most relevant experience. By continuing to browse the site you are agreeing to our use of cookies.