Tags | |
UUID | 088c59af-2cd3-11e6-9770-bc764e2038f2 |
The absorbance equation calculates the amount of energy absorbed by a molecule when the molecule is excited by a photon, and is promoted from the ground state (lowest energy level) to an excited state. This is demonstrated in an image below.
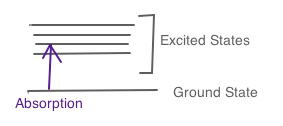
Description
The equation is
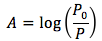
where
- P0 is initial irradiance (or intensity of light) from wavelength selector to sample in units of (Energy/sec*area of light beam)
- P is the irradiance from sample to the light detector in units of (Energy/sec*area of light beam)
- A is absorbance
Related Topics
- Beer's Law (for the Spanish site click here)
- Transmittance (for the Spanish site click here)
References
Harris, 9th Edition. Pp.435
This equation, Absorbance, is used in 1 page
Collections
- Comments
- Attachments
- Stats
No comments |
This site uses cookies to give you the best, most relevant experience. By continuing to browse the site you are agreeing to our use of cookies.
