Tags | |
UUID | 63032888-38ac-11e6-9770-bc764e2038f2 |
This region I titration equation calculates the pH of the solution before the strong base is added to the weak acid.
Solving the titration problem for Region I: Before base is added.
Given: Formal concentration of weak acid and strong base, Ka or pKa.
Since the strong base is not added yet, the solution contains only water and the weak acid. Thus we can treat this as a a typical acid equilibrium problem and solve for the hydronium ion concentration to find the pH.
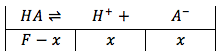
the equilibrium equation is then
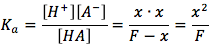
* Note: we can ignore the x in the denominator, since F>>x, we can assume that the hydronium concentration will not have a significant affect on the formal concentration.
We then multiply both sides by F and take the square root and get the following equation:
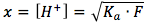
we can then plug this in to the pH equation, then the final equation is
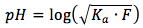
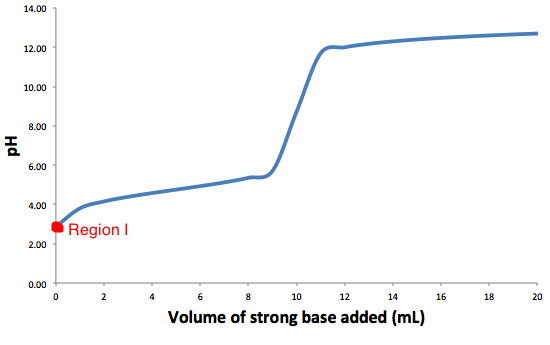
This is a typical graph for titration of weak acid with a strong base.
Description
The equation is
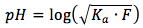
where
- Ka is the acid equilibrium constant, the constants for all of the acids can be found here.
- F is the formal concentration of the weak acid in units of (mol/L)
References
Harris, 9th Edition. Pp.236-238
Calculators
Collections
- Comments
- Attachments
- Stats
No comments |
