Tags | |
UUID | db249ca2-31c2-11e6-9770-bc764e2038f2 |
The equation for weak acid is used to calculated the unknown concentration of weak acid after dissociation in solution. This equation is paired with fraction of dissociation of an acid equation.
The equation is derived from
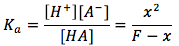 where the original weak acid dissociation reaction is
where the original weak acid dissociation reaction is 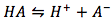 .
.
Solving for x , we assume that x is going to be a very small number thus (F-x)=F. Then the equation becomes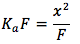 . Multiplying both sides by F (formal concentration), we get
. Multiplying both sides by F (formal concentration), we get 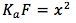 . Taking the square root of both sides we get
. Taking the square root of both sides we get 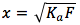 .
.
Description
The equation is
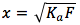
where
- x is the [A-] concentration of the dissociated acid particle in units of (mol/L)
- F is the formal concentration in units of (mol/L)
- Ka is the acid dissociation constant, an extended list can be found here.
Related Topics
- Fraction dissociation for weak acid (for the Spanish site click here)
- Acid equilibrium constant (for the Spanish site click here)
References
Harris, 9th Edition. Pp.193
This equation, Equation for weak acid, is used in 1 page
Collections
- Comments
- Attachments
- Stats
No comments |
This site uses cookies to give you the best, most relevant experience. By continuing to browse the site you are agreeing to our use of cookies.
