Fraction dissociation for weak base
Tags | |
UUID | e6f73fac-2c63-11e6-9770-bc764e2038f2 |
The Fraction Dissociation for Weak Base calculator computes the fractional dissociation based on the concentration of the Base Dissociation Concentration and Formal Concentration.
INSTRUCTIONS: Choose units and enter the following:
- [BH+] Base Dissociation Concentration
- [F] Formal Concentration
Fraction Dissociation for Weak Base (α): The calculator returns the dissociation as a real number.
The Math / Science
The fraction dissociation for weak-base calculates the fraction (or the percentage) of weak base that has reacted with water.
The equation is created using a mass balance equation and algebraic substitution in order to simplify the number of unknowns. The mass balance equation is
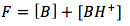
we can rearrange the mass balance equation to solve for [B],

We can substitute this in to the fraction of dissociation equation and we get
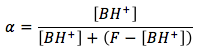
This way we only have one unknown, by letting [BH+] equal x, we get the final equation
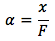
Description
The equation is
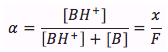
where
- x is the concentration of the dissociated weak-base [BH+] in units of (mol/L)
- F is the formal concentration in units of (mol/L)
- Alpha is the fraction of dissociation (unit-less)
References
Harris, 9th Edition. Pp.195
Collections
- Comments
- Attachments
- Stats
No comments |
