Charge balance
n1[C1]+n2[C2]+...=m1[A1]+m2[A2]+...
(C1)Molar concentration of cation species 1 | ||
(n1)Charge of cation species 1 | ||
(m1)Charge of anion species 1 | ||
(A1)Molar concentration of an anion species 1 | ||
(n2)Charge of cation species 2 | ||
(C2)Molar concentration of cation species 2 | ||
(m2)Charge of anion species 2 | ||
(A2)Molar concentration of an anion species 2 | ||
Tags | |
UUID | fca066f7-2f32-11e6-9770-bc764e2038f2 |
The charge balance equation is used to solve for a missing concentration of an ion. This neutrality equation is designed in a way where the molarity concentrations of cations along with their charge coefficients equal the molarity concentrations of anions and their charge coefficients.
Description
The equation is
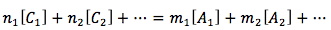
or in summation form
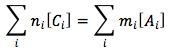
where
- ni is the ith species of cation charge coefficient.
- Ci is the ith species of cation molar concentration in units of (mol/L)
- mi is the ith species of an anion charge coefficient.
- Ai is the ith species of an anion molar concentration in units of (mol/L)
*Notice: Both sides must equal each other, this equation was programmed so that the subtraction of cations from an anions was 0.
Related Topics
- Mass Balance
- Balance de Masa (in Spanish)
References
Harris, 9th Edition. Pp.170
This equation, Charge balance, is used in 1 page
Collections
- Comments
- Attachments
- Stats
No comments |
This site uses cookies to give you the best, most relevant experience. By continuing to browse the site you are agreeing to our use of cookies.
