Processing...
`[BH^+] = sqrt( F * Kb )`
Enter a value for all fields
The equation for weak base calculator calculates the unknown concentration of the dissociated weak base in a water solution.
INSTRUCTIONS: Choose the preferred units and enter the following:
- (Kb) This is the base equilibrium constant
- (F) This is the formal concentration in units of (mol/L)
Concentration of Dissociated Base: Thee concentration of the dissociated base [BH+] in units of (mol/L)
The Math / Science
The common weak-base reaction is
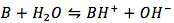
for which the equilibrium equation is
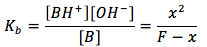
since we are usually given the formal concentration , the [BH+]=x is unknown and we must solve for it. First we assume that x is going to be much smaller than the formal concentration, thus the equilibrium equation becomes
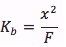
Multiplying both sides by the formal concentration we get
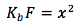
Taking a square root of both sides we get
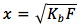
Description
The equation is
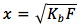
where
- Kb is the base equilibrium constant
- F is the formal concentration in units of (mol/L)
- x is the concentration of the dissociated base [BH+] in units of (mol/L)
References
Harris, 9th Edition. Pp.195