Processing...
- A The K+ cation has a small positive charge (+1) and a relatively large radius (because it is in the fourth row of the periodic table), so it is a very weak Lewis acid.
B The NO3? anion is the conjugate base of a strong acid, so it has essentially no basic character. Hence neither the cation nor the anion will react with water to produce H+ or OH?, and the solution will be neutral.
- A The Cr3+ ion is a relatively highly charged metal cation that should behave similarly to the Al3+ ion and form the [Cr(H2O)6]3+ complex, which will behave as a weak acid:
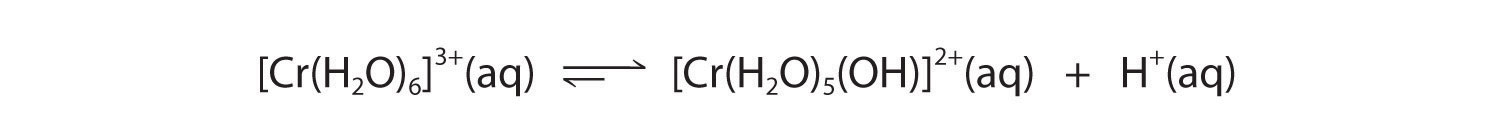
B The Br? anion is a very weak base (it is the conjugate base of the strong acid HBr), so it does not affect the pH of the solution. Hence the solution will be acidic.
- A The Na+ ion, like the K+, is a very weak acid, so it should not affect the acidity of the solution.
B In contrast, SO42? is the conjugate base of HSO4?, which is a weak acid. Hence the SO42? ion will react with water as shown in to give a slightly basic solution.