Qualitative Description of Acid–Base Equilibriums
From UCDavis Chemwiki and UCDavis Chemwiki
We now turn our attention to acid–base reactions to see how the concepts of chemical equilibrium and equilibrium constants can deepen our understanding of this kind of chemical behavior. We begin with a qualitative description of acid–base equilibriums in terms of the Brønsted–Lowry model and then proceed to a quantitative description in the next section"Quantitative Aspects of Acid–Base Equilibriums".
Conjugate Acid–Base Pairs
We discussed the concept of conjugate acid–base pairs in a previous section, using the reaction of ammonia, the base, with water, the acid, as an example. In aqueous solutions, acids and bases can be defined in terms of the transfer of a proton from an acid to a base. Thus for every acidic species in an aqueous solution, there exists a species derived from the acid by the loss of a proton. These two species that differ by only a proton constitute a conjugate acid–base pair. For example, in the reaction of HCl with water, HCl, the parent acid, donates a proton to a water molecule, theparent base, thereby forming Cl-. Thus HCl and Cl- constitute a conjugate acid–base pair. By convention, we always write a conjugate acid–base pair as the acid followed by its conjugate base. In the reverse reaction, the Cl- ion in solution acts as a base to accept a proton from H3O+, forming H2O and HCl. Thus H3O+ and H2O constitute a second conjugate acid–base pair. In general, any acid–base reaction must contain two conjugate acid–base pairs, which in this case are HCl/Cl- and H3O+/H2O.
Note the Pattern
All acid–base reactions contain two conjugate acid–base pairs.
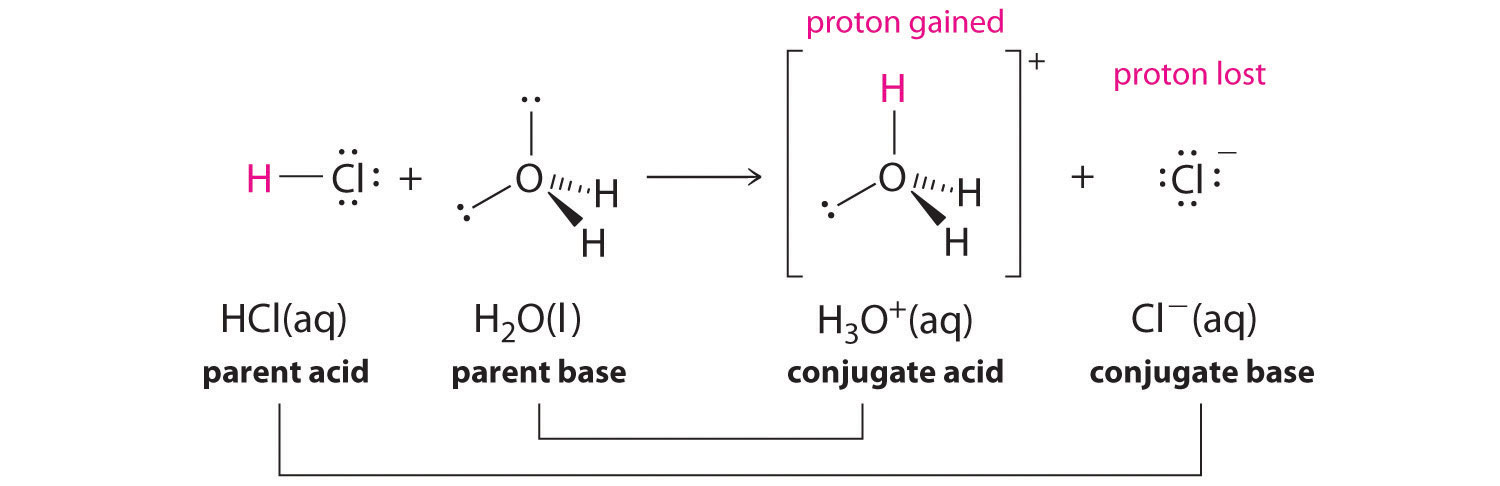
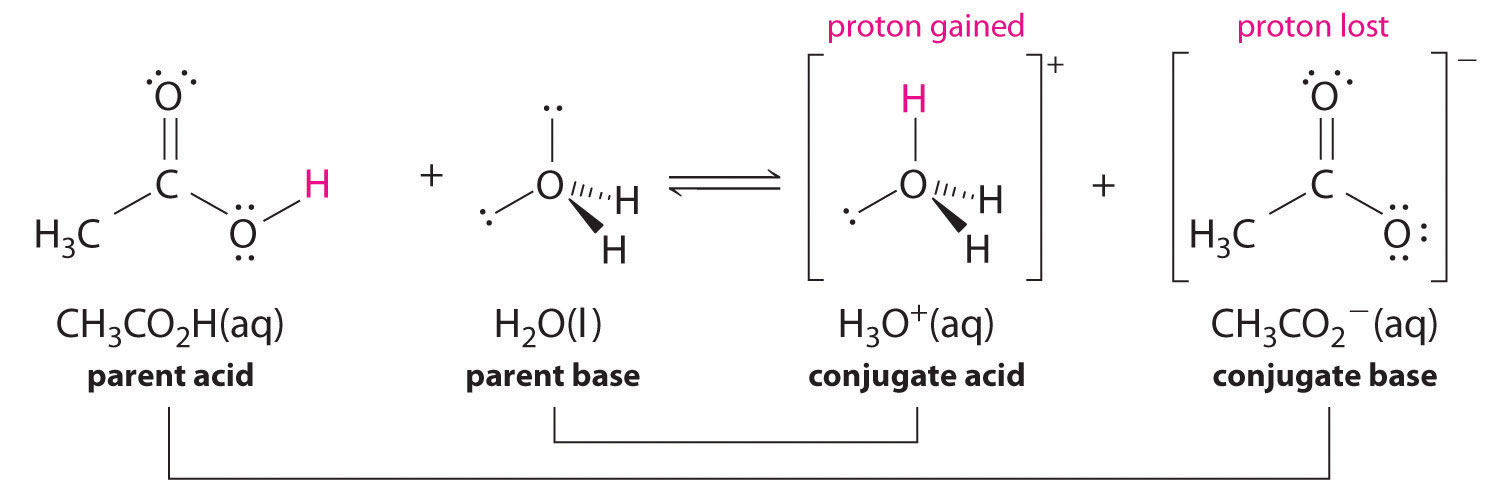
Similarly, in the reaction of acetic acid with water, acetic acid donates a proton to water, which acts as the base. In the reverse reaction, H3O+ is the acid that donates a proton to the acetate ion, which acts as the base. Once again, we have two conjugate acid–base pairs: the parent acid and its conjugate base (CH3CO2H/CH3CO2-) and the parent base and its conjugate acid (H3O+/H2O).
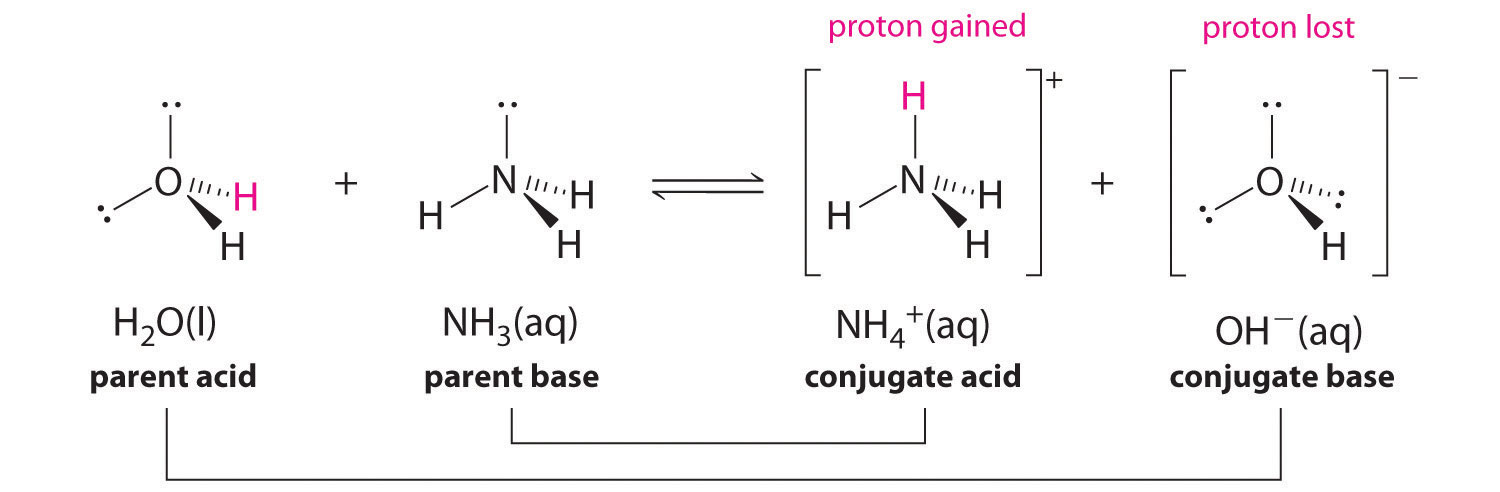
In the reaction of ammonia with water to give ammonium ions and hydroxide ions (Equation 16.3), ammonia acts as a base by accepting a proton from a water molecule, which in this case means that water is acting as an acid. In the reverse reaction, an ammonium ion acts as an acid by donating a proton to a hydroxide ion, and the hydroxide ion acts as a base. The conjugate acid–base pairs for this reaction are NH4+/NH3 and H2O/OH-. Some common conjugate acid–base pairs are shown here.
The Relative Strengths of Some Common Conjugate Acid–Base Pairs
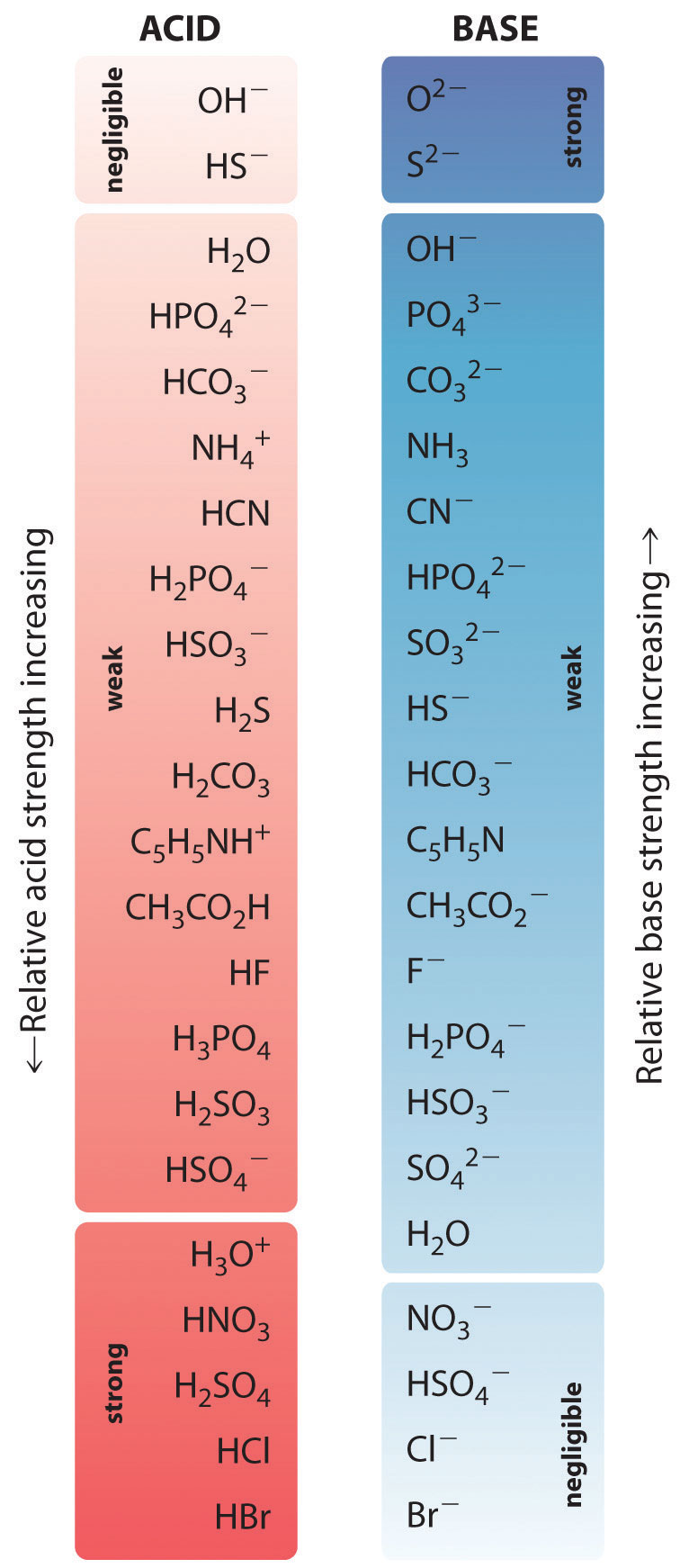
]The strongest acids are at the bottom left, and the strongest bases are at the top right. The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid.
Acid–Base Equilibrium Constants: Ka, Kb, pKa, and pKb
The magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids and bases. For example, the general equation for the ionization of a weak acid in water, where HA is the parent acid and A- is its conjugate base, is as follows:
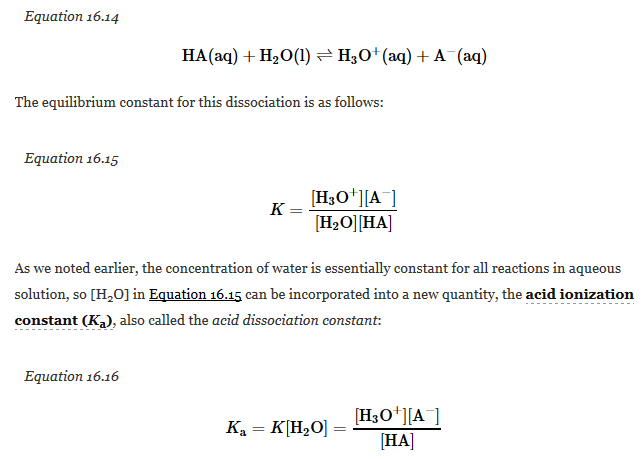
Thus the numerical values of K and Ka differ by the concentration of water (55.3 M). Again, for simplicity, H3O+ can be written as H+. Keep in mind, though, that free H+ does not exist in aqueous solutions and that a proton is transferred to H2O in all acid ionization reactions to form H3O+. The larger the Ka, the stronger the acid and the higher the H+concentration at equilibrium.Like all equilibrium constants, acid–base ionization constants are actually measured in terms of the activities of H+ or OH-, thus making them unitless. The values of Ka for a number of common acids are given in the following table.
Values of Ka, pKa, Kb, and pKb for Selected Acids (HA) and Their Conjugate Bases (A?)
| Acid | HA | Ka | pKa | A- | Kb | `pK_b` |
|---|---|---|---|---|---|---|
| hydroiodic acid | HI | 2×109 | -9.3 | I^(-) | 5.5×10-24 | 23.26 |
| sulfuric acid (1)* | H2SO4 | 1×102 | -2.0 | HSO-4 | 1×10-16 | 16.0 |
| nitric acid | HNO3 | 2.3×101 | -1.37 | NO-3 | 4.3×10-16 | 15.37 |
| hydronium ion | H3O+ | 1.0 | 0.00 | H2O | 1.0×10-14 | 14.00 |
| sulfuric acid (2)* | HSO-4 | 1.0×10-2 | 1.99 | SO2-4 | 9.8×10-13 | 12.01 |
| hydrofluoric acid | HF | 6.3×10-4 | 3.20 | F- | 1.6×10-11 | 10.80 |
| nitrous acid | HNO2 | 5.6×10-4 | 3.25 | `NO_2^(-) | 1.8×10-11 | `10.75 |
| formic acid | HCO2H | 1.78×10-4 | 3.750 | HCO-2 | 5.6×10-11 | 10.25 |
| benzoic acid | C6H5CO2H | 6.3×10-5 | 4.20 | C6H5CO-2 | 1.6×10-10 | 9.80 |
| acetic acid | CH3CO2H | 1.7×10-5 | 4.76 | CH3CO-2 | 5.8×10-10 | 9.24 |
| pyridinium ion | `C_5H_5NH^(+) | 5.9×10-6 | 5.23 | C-5H5N | 1.7×10-9 | 8.77 |
| hypochlorous acid | HOCl | 4.0×1-8 | 7.40 | OCl- | 2.5×10-7 | 6.60 |
| hydrocyanic acid | HCN | 6.2×1-10 | 9.21 | CN- | 1.6×10-5 | 4.79 |
| ammonium ion | NH+4 | 5.6×1-10 | 9.25 | NH3 | 1.8×10-5 | 4.75 |
| water | H2O | 1.0×1-14 | 14.00 | OH- | 1.00 | 0.00 |
| acetylene | C2H2 | 1×1-26 | 26.0 | HC-2 | 1×1012 | -12.0 |
| ammonia | NH3 | 1×1-35 | 35.0 | NH-2 | 1×1021 | -21.0 |
| *The number in parentheses indicates the ionization step referred to for a polyprotic acid. | ||||||
Weak bases react with water to produce the hydroxide ion, as shown in the following general equation, where B is the parent base and BH+ is its conjugate acid:
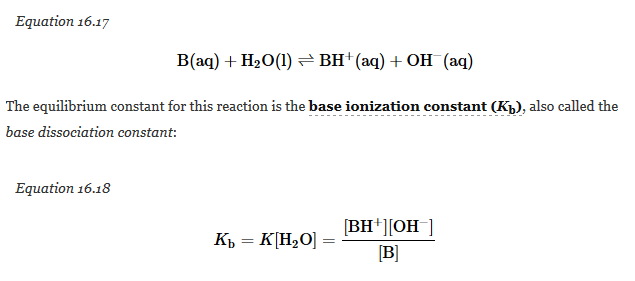
Once again, the concentration of water is constant, so it does not appear in the equilibrium constant expression; instead, it is included in the Kb. The larger the Kb, the stronger the base and the higher the OH- concentration at equilibrium. The values of Kb for a number of common weak bases are given in the following table.
Values of Kb, pKb, Ka, and pKa for Selected Weak Bases (B) and Their Conjugate Acids (BH+)
| Base | B | K b | pKb | BH+ | K a | pKa |
|---|---|---|---|---|---|---|
| hydroxide ion | `OH^(-) | 1.0 | 0.00⋅ | H2O | 1.0×10-14 | 14.00 |
| phosphate ion | PO3-4 | 2.1×10-2 | 1.68 | HPO2-4 | 4.8×10-13 | 12.32 |
| dimethylamine | (CH3)2NH | 5.4×10-4 | 3.27 | (CH3)2NH+2 | 1.9×10-11 | 10.73 |
| methylamine | CH3NH2 | 4.6×10-4 | 3.34 | CH3NH+3 | 2.2×10-11 | 10.66 |
| trimethylamine | (CH3)3N | 6.3×10-5 | 4.20 | `(CH_3)_3NH^(+) | 1.6×10-10 | 9.80 |
| ammonia | NH3 | 1.8×10-5 | 4.75 | NH+4 | 5.6×10-10 | 9.25 |
| pyridine | C5H5N | 1.7×10-9 | 8.77 | C5H5NH+ | 5.9×10-6 | 5.23 |
| aniline | C6H5NH2 | 7.4×10-10 | 9.13 | C6H5NH+3 | 1.3×1)-5 | 4.87 |
| water | H2O | 1.0×10-14 | 14.00 | H3O+ | 1.0⋅ | 0.00 |
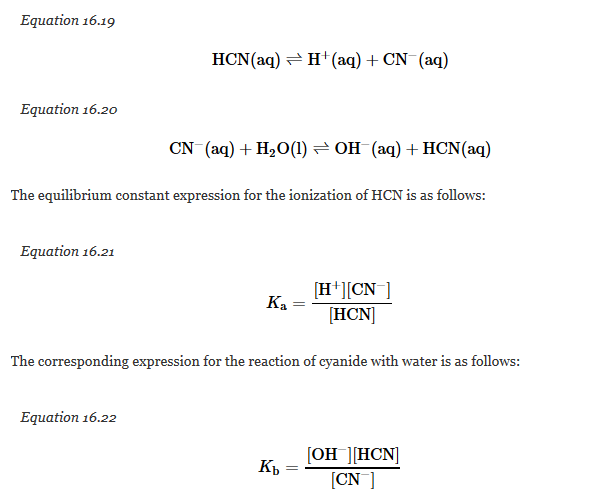
There is a simple relationship between the magnitude of Ka for an acid and Kb for its conjugate base. Consider, for example, the ionization of hydrocyanic acid (HCN) in water to produce an acidic solution, and the reaction of CN? with water to produce a basic solution:
HCN(aq) Equation 1
CN^(-) (aq) + H_2 O(l) ? OH^(-) (aq) + HCN (aq) Equation 2
The equilibrium constant expression for the ionization of HCN is as follows:
K_a = ([H^(+)][CN^(-)])/([HCN]) Equation 3
The corresponding expression for the reaction of cyanide with water is as follows:
K_b = ([OH^(-)][HCN])/([CN^(-)]) Equation 4
If we add Equation 16.19 and Equation 16.20, we obtain the following:
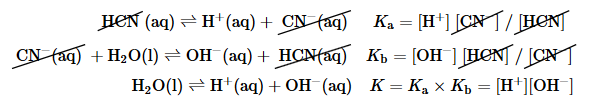
In this case, the sum of the reactions described by Ka and Kb is the equation for the autoionization of water, and the product of the two equilibrium constants is Kw:
Equation 16.23
KaKb = KwThus if we know either Ka for an acid or Kb for its conjugate base, we can calculate the other equilibrium constant for any conjugate acid–base pair.
Just as with pH, pOH, and pKw, we can use negative logarithms to avoid exponential notation in writing acid and base ionization constants, by defining pKa as follows:
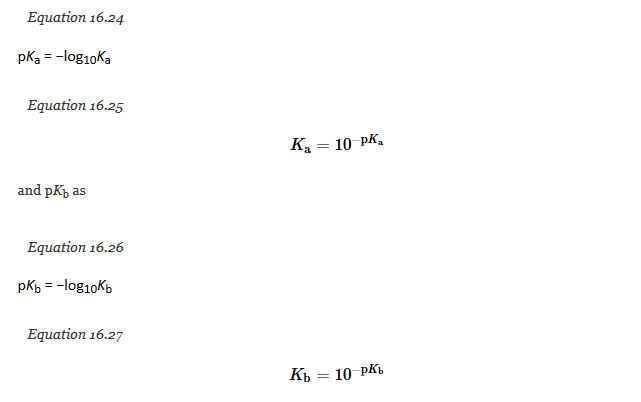
Similarly, Equation 16.23, which expresses the relationship between Ka and Kb, can be written in logarithmic form as follows:
Equation 16.28
pKa + pKb = pKwAt 25°C, this becomes
Equation 16.29
pKa + pKb = 14.00The relative strengths of some common acids and their conjugate bases are shown graphically in the figure above "The Relative Strengths of Some Common Conjugate Acid–Base Pairs". The conjugate acid–base pairs are listed in order (from top to bottom) of increasing acid strength, which corresponds to decreasing values of pKa. This order corresponds to decreasing strength of the conjugate base or increasing values of pKb. At the bottom left of the figure are the common strong acids; at the top right are the most common strong bases. Notice the inverse relationship between the strength of the parent acid and the strength of the conjugate base. Thus the conjugate base of a strong acid is a very weak base, and the conjugate base of a very weak acid is a strong base.
Note the Pattern
The conjugate base of a strong acid is a weak base and vice versa.
We can use the relative strengths of acids and bases to predict the direction of an acid–base reaction by following a single rule: an acid–base equilibrium always favors the side with the weaker acid and base, as indicated by these arrows:
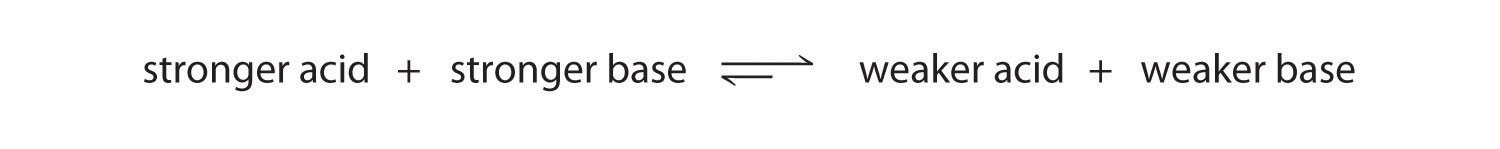
In an acid–base reaction, the proton always reacts with the stronger base.
For example, hydrochloric acid is a strong acid that ionizes essentially completely in dilute aqueous solution to produce H3O+ and Cl-; only negligible amounts of HCl molecules remain undissociated. Hence the ionization equilibrium lies virtually all the way to the right, as represented by a single arrow:
Equation 16.30
HCl (aq) + H2O (l) --> H3O+ (aq) + Cl- (aq)
In contrast, acetic acid is a weak acid, and water is a weak base. Consequently, aqueous solutions of acetic acid contain mostly acetic acid molecules in equilibrium with a small concentration of H3O+ and acetate ions, and the ionization equilibrium lies far to the left, as represented by these arrows:
Figure 16.3
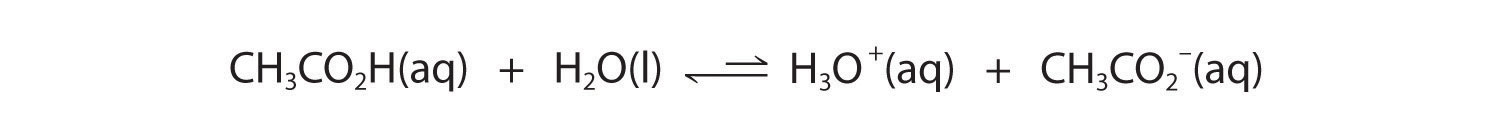
Similarly, in the reaction of ammonia with water, the hydroxide ion is a strong base, and ammonia is a weak base, whereas the ammonium ion is a stronger acid than water. Hence this equilibrium also lies to the left:
Figure 16.4
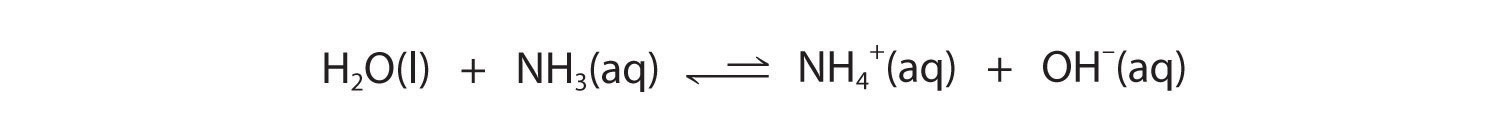
Note the Pattern
All acid–base equilibriums favor the side with the weaker acid and base. Thus the proton is bound to the stronger base.
Example 4
- Calculate Kb and pKb of the butyrate ion (CH3CH2CH2CO2-). The pKa of butyric acid at 25°C is 4.83. Butyric acid is responsible for the foul smell of rancid butter.
- Calculate Ka and pKa of the dimethylammonium ion [(CH3)2NH2+]. The base ionization constant Kb of dimethylamine [(CH3)2NH] is 5.4 × 10-4 at 25°C.
 Subpages (1): Example 4
Subpages (1): Example 4