Tags | |
UUID | 19ef559e-f145-11e9-8682-bc764e2038f2 |
Buffers
From UCDavis Chemwiki
|
A buffer is a solution that can resist pH change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus maintaining the pH of the solution relatively stable. This is important for processes and/or reactions which require specific and stable pH ranges. Buffer solutions have a working pH range and capacity which dictate how much acid/base can be neutralized before pH changes, and the amount by which it will change. What is a buffer composed of?To effectively maintain a pH range, a buffer must consist of a weak conjugate acid-base pair, meaning either a. a weak acid and its conjugate base, or b. a weak base and its conjugate acid. The use of one or the other will simply depend upon the desired pH when preparing the buffer. For example, the following could function as buffers when together in solution:
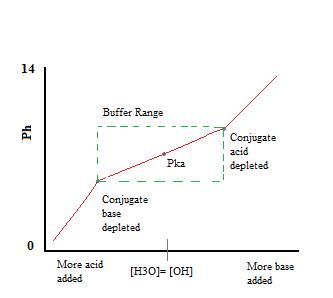
How does a buffer work?A buffer is able to resist pH change because the two components (conjugate acid and conjugate base) are both present in appreciable amounts at equilibrium and are able to neutralize small amounts of other acids and bases (in the form of H3O+ and OH-) when the are added to the solution. In order to clarify this effect, we can consider the simple example of a Hydrofluoric Acid (HF) and Sodium Fluoride (NaF) buffer. Hydrofluoric acid is a weak acid due to the strong attraction between the relatively small F- ion and solvated protons (H3O+), which does not allow it to dissociate completely in water. Therefore, if we obtain HF in an aqueous solution, we establish the following equilibrium with only slight dissociation (Ka(HF) = 6.6x10-4, strongly favors reactants): HF(aq)+H2O(l) ?F?(aq)+H3O+(aq)We can then add and dissolve sodium fluoride into the solution and mix the two until we reach the desired volume and pH at which we want to buffer. When Sodium Fluoride dissolves in water, the reaction goes to completion, thus we obtain: NaF(aq)+H2O(l) ?Na+(aq)+F?(aq)Since Na+ is the conjugate of a strong base, it will have no effect on the pH or reactivity of the buffer. The addition of NaF to the solution will, however, increase the concentration of F- in the buffer solution, and, consequently, by Le Chatlier's principle, lead to slightly less dissociation of the HF in the previous equilibrium, as well. The presence of significant amounts of both the conjugate acid, HF, and the conjugate base, F-, allows the solution to function as a buffer. This buffering action can be seen in the titration curve of a buffer solution.
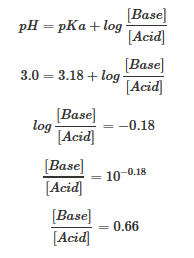
As we can see, over the working range of the buffer. pH changes very little with the addition of acid or base. Once the buffering capacity is exceeded the rate of pH change quickly jumps. This occurs because the conjugate acid or base has been depleted through neutralization. This principle implies that a larger amount of conjugate acid or base will have a greater buffering capacity. If acid is added: F?(aq)+H3O+(aq) ? HF(aq)+H2O(l)In this reaction, the conjugate base, F-, will neutralize the added acid, H3O+, and this reaction goes to completion, because the reaction of F- with H3O+ has an equilibrium constant much greater than one. (In fact, the equilibrium constant the reaction as written is just the inverse of the Ka for HF: 1/Ka(HF) = 1/(6.6x10-4) = 1.5x10+3.) So long as there is more F- than H3O+, almost all of the H3O+ will be consumed and the equilibrium will shift to the right, slightly increasing the concentration of HF and slightly decreasing the concentration of F-, but resulting in hardly any change in the amount of H3O+ present once equilibrium is re-established. If base is added: HF(aq)+OH?(aq) ? F?(aq)+H2O(l)In this reaction, the conjugate acid, HF, will neutralize added amounts of base, OH-, and the equilibrium will again shift to the right, slightly increasing the concentration of F- in the solution and decreasing the amount of HF slightly. Again, since most of the OH- is neutralized, little pH change will occur. These two reactions can continue to alternate back and forth with little pH change. Selecting proper components for desired pHBuffers function best when the pKa of the conjugate weak acid used is close to the desired working range of the buffer. This turns out to be the case when the concentrations of the conjugate acid and conjugate base are approximately equal (within about a factor of 10). For example, we know the Ka for hydroflouric acid is 6.6 x 10-4 so its pKa= -log(6.6 x 10-4) = 3.18. So, a hydrofluoric acid buffer would work best in a buffer range of around pH = 3.18. For the weak base ammonia (NH3), the value of Kb is 1.8x10-5, implying that the Ka for the dissociation of its conjugate acid, NH4+, is Kw/Kb=10-14/1.8x10-5 = 5.6x10-10. Thus, the pKa for NH4+ = 9.25, so buffers using NH4+/NH3 will work best around a pH of 9.25. (It's always the pKa of the conjugate acid that determines the approximate pH for a buffer system, though this is dependent on the pKb of the conjugate base, obviously.) When the desired pH of a buffer solution is near the pKa of the conjugate acid being used (i.e., when the amounts of conjugate acid and conjugate base in solution are within about a factor of 10 of each other), the Henderson-Hasselbalch equation can be applied as a simple approximation of the solution pH, as we will see in the next section.
Adding Strong Acids or Bases to Buffer SolutionsNow that we have this nice F-/HF buffer, let's see what happens when we add strong acid or base to it. Recall that the amount of F- in the solution is 0.66M x 0.1 L = 0.066 moles and the amount of HF is 1.0 M x 0.1L = 0.10 moles. Let's double check the pH using the Henderson-Hasselbalch Approximation, but using moles instead of concentrations: pH = pKa + log(Base/Acid) = 3.18 + log(0.066 moles F-/0.10 moles HF) = 3.00 Good. Now let's see what happens when we add a small amount of strong acid, such as HCl. When we put HCl into water, it completely dissociates into H3O+ and Cl-. The Cl- is the conjugate base of a strong acid so is inert and doesn't affect pH, and we can just ignore it. However, the H3O+ can affect pH and it can also react with our buffer components. In fact, we already discussed what happens. The equation is: F?(aq)+H3O+(aq) ? HF(aq)+H2O(l)For every mole of H3O+ added, an equivalent amount of the conjugate base (in this case, F-) will also react, and the equilibrium constant for the reaction is large, so the reaction will continue until one or the other is essentially used up. If the F- is used up before reacting away all of the H3O+, then the remaining H3O+ will affect the pH directly. In this case, the capacity of the buffer will have been exceeded - a situation one tries to avoid. However, for our example, let's say that the amount of added H3O+ is smaller than the amount of F- present, so our buffer capacity is NOT exceeded. For the purposes of this example, we'll let the added H3O+ be equal to 0.01 moles (from 0.01 moles of HCl). Now, if we add 0.01 moles of HCl to 100 mL of pure water, we would expect the pH of the resulting solution to be 1.00 (0.01 moles/0.10 L = 0.1 M; pH = -log(0.1) = 1.0). However, we are adding the H3O+ to a solution that has F- in it, so the H3O+ will all be consumed by reaction with F-. In the process, the 0.066 moles of F- is reduced: 0.066 initial moles F- - 0.010 moles reacted with H3O+ = 0.056 moles F- remaining Also during this process, more HF is formed by the reaction: 0.10 initial moles HF + 0.010 moles from reaction of F- with H3O+ = 0.11 moles HF after reaction Plugging these new values into Henderson-Hasselbalch gives: pH = pKa + log (base/acid) = 3.18 + log (0.056 moles F-/0.11 moles HF) = 2.89 Thus, our buffer did what it should - it resisted the change in pH, dropping only from 3.00 to 2.89 with the addition of 0.01 moles of strong acid. Example 2 What is the pH of a solution that contains
Example 3 The buffer solution in Example 15 contained 0.135 M HCO2H and 0.215 M HCO2Na and had a pH of 3.95.
|
This Collection is empty
Collections
- Comments
- Attachments
- Stats
No comments |