Tags | |
UUID | 19f2384d-f145-11e9-8682-bc764e2038f2 |
Weak Acid/Strong Base Titrations
From UCDavis Chemwiki
| vCalc Companion Formulas | |
| vCalc Formulary | CHM1 18 Weak Acid Strong Base Titration |
| pH=pKa+(log([A-])[HA]) | Henderson Hasselbalch Equation |
In this reaction a buret is used to administer one solution to another. The solution administered from the buret is called the titrant. The solution that the titrant is added to is called the analyte. In a titration of a Weak Acid with a Strong Base the titrant is a strong base and the analyte is a weak acid. In order to fully understand this type of titration the reaction, titration curve, and type of titration problems will be introduced.
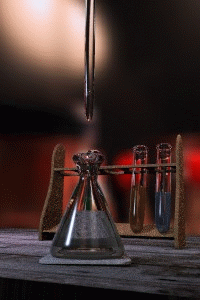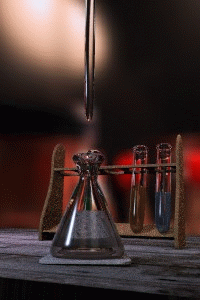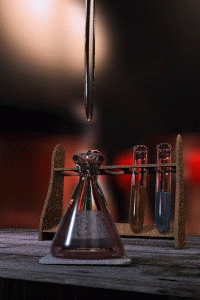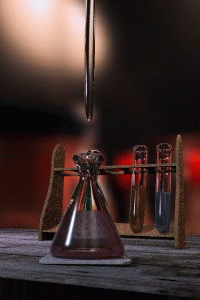
Figure 1: Titrations involve the addition of the titrant from the burret to the analyte. Figure is used with the permission of J.A. Freyre under the Creative Commons Attributions-Share Alike 2.5 Generic
The Reaction
The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The reaction of the weak acid, oxalic acid, with a strong base, NaOH, can be seen below. In the reaction the acid and base react in a one to one ratio.
H2C2O4 + OH- ? H2O + HC2O4-
The Titration Curve
The titration curve is a graph of the volume of titrant, or in our case the volume of strong base, plotted against the pH. There are several characteristics that are seen in all titration curves of a weak acid with a strong base. These characteristics are stated below.
- The initial pH (before the addition of any strong base) is higher or less acidic than the titration of a strong acid
- There is a sharp increase in pH at the beginning of the titration. This is because the anion of the weak acid becomes a common ion that reduces the ionization of the acid.
- After the sharp increase at the beginning of the titration the curve only changes gradually. This is because the solution is acting as a buffer. This will continue until the base overcomes the buffers capacity.
- In the middle of this gradually curve the half-neutralization occurs. At this point the concentration of weak acid is equal to the concentration of its conjugate base. Therefore the pH=pKa. This point is called the half-neutralization because half of the acid has been neutralized.
- At the equivalence point the pH is greater then 7 because all of the acid (HA) has been converted to its conjugate base (A-) by the addition of NaOH and now the equilibrium moves backwards towards HA and produces hydroxide, that is:
A-+H2O ? AH + OH-
6. The steep portion of the curve prior to the equivalence point is short. It usually only occurs at a pH of around 10.The image of a titration curve of a weak acid with a strong base is seen below. All of the characteristics described above can be seen within it.
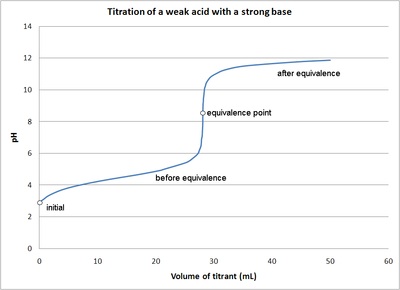
Figure 2: The titration of a weak acid with strong base. Figure is used with the permission of J.A. Freyre under the Creative Commons Attributions-Share Alike 2.5 Generic
Weak Acid and Strong Base Titration Problems
When solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. These include the initial pH, the pH after adding a small amount of base, the pH at the half-neutralization, the pH at the equivalence point, and finally the pH after adding excess base. This data will give sufficient information about the titration. Below is an example of this process.
Find the pH at each of the following points in the titration of 25 mL of 0.3 M HF with 0.3 M NaOH. The ka value is 6.6*10-4
- The initial pH
- After adding 10 mL of 0.3 M NaOH
- After adding 12.50 mL of 0.3 M NaOH
- After adding 25 mL of 0.3 M NaOH
- After adding 26 mL of 0.3 M NaOH
| Example 9: The initial pH | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Finding the initial pH. SOLUTION Since HF is a weak acid, the use of an ICE table is required to find the pH. The question gives us the concentration of the HF.
|
||||||||||||||||||||
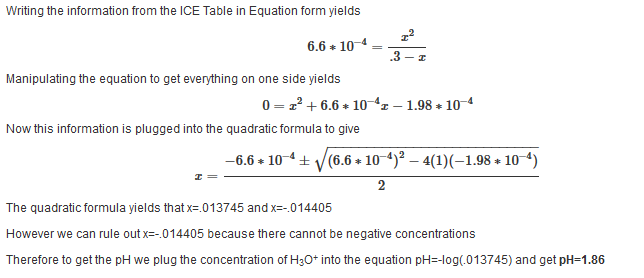
| Example 10: After adding 10 mL of 0.3 M NaOH |
|---|
| Find the pH after the addition of 10 mL of 0.3 M NaOH. |
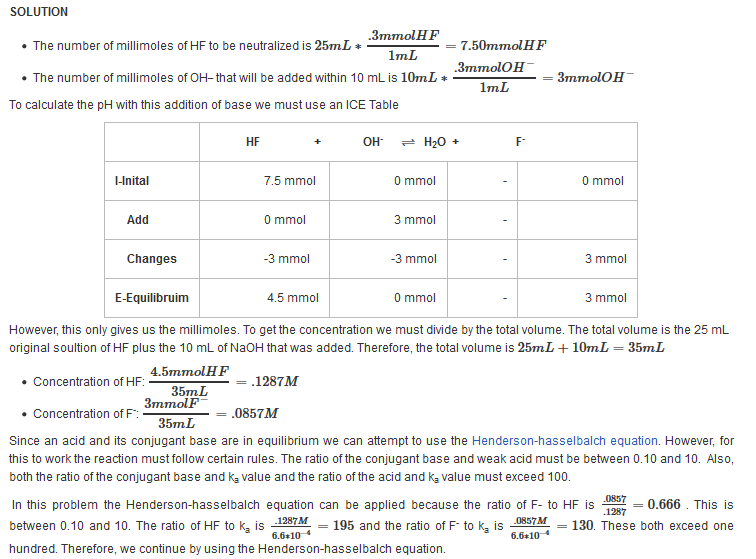
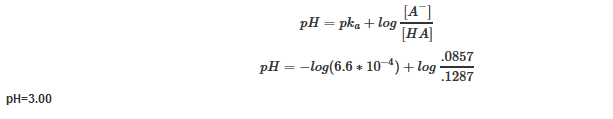
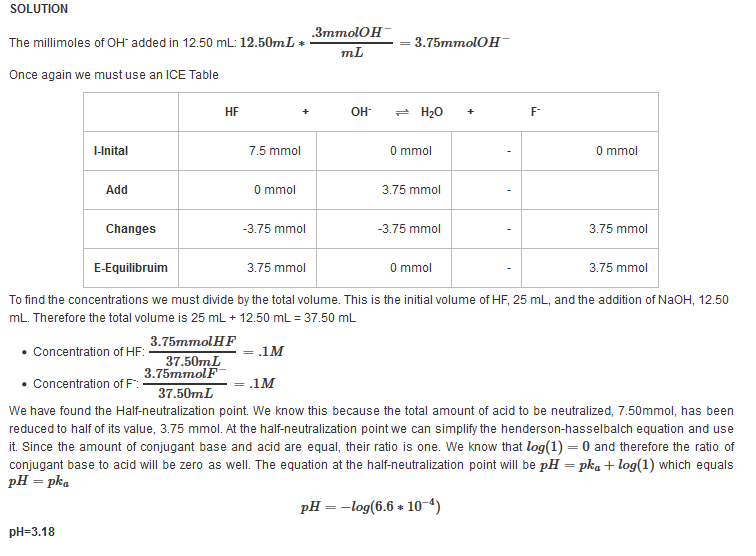
| Example 11: After adding 12.50 mL of 0.3 M NaOH |
|---|
| Find the pH after adding 12.50 mL of 0.3 M NaOH |
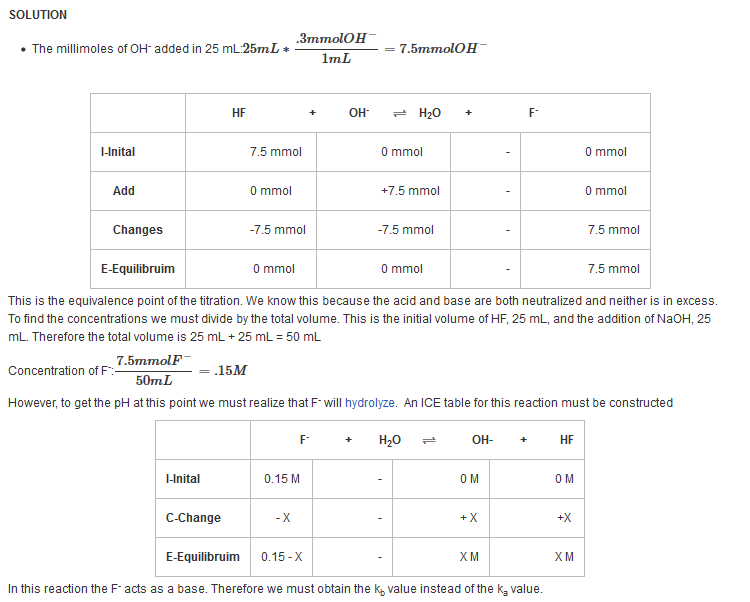
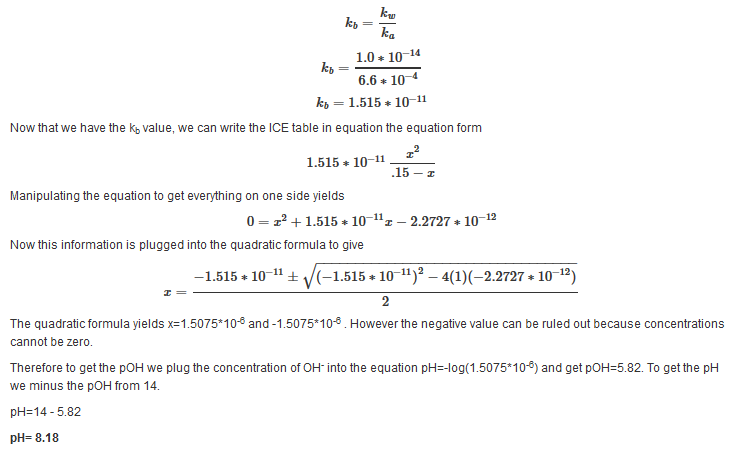
| Example 12: After adding 25 mL of 0.3 M NaOH |
|---|
| Find the pH after the addition of 25 mL of NaOH. |
| Example 13: After adding 26 mL of 0.3 M NaOH |
|---|
|
Find the pH after the addition of 26 mL of NaOH.
The titration curves for weak bases and strong acids are similar to those for weak acids and strong bases except that they are inverted (recall that strong is added to weak). |
This Collection is empty
- Comments
- Attachments
- Stats
No comments |

