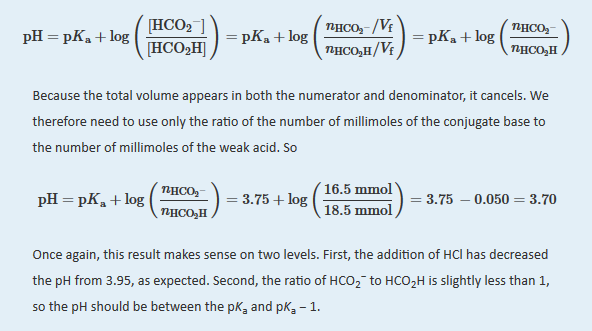The added HCl (a strong acid) or NaOH (a strong base) will react completely with formate (a weak base) or formic acid (a weak acid), respectively, to give formic acid or formate and water. We must therefore calculate the amounts of formic acid and formate present after the neutralization reaction.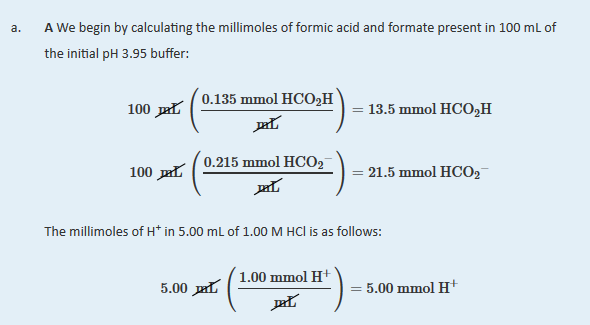
Next, we construct a table of initial amounts, changes in amounts, and final amounts:
| HCO2?(aq) + H+(aq) ? HCO2H(aq) | |||
|---|---|---|---|
| [HCO2?] | [H+] | [HCO2H] | |
| initial | 21.5 mmol | 5.00 mmol | 13.5 mmol |
| change | ?5.00 mmol | ?5.00 mmol | +5.00 mmol |
| final | 16.5 mmol | ?0 mmol | 18.5 mmol |
The final amount of H+ in solution is given as “?0 mmol.” For the purposes of the stoichiometry calculation, this is essentially true, but remember that the point of the problem is to calculate the final [H+] and thus the pH. We now have all the information we need to calculate the pH. We can use either the lengthy procedure of Example 14 or the Henderson–Hasselbach equation. Because we have performed many equilibrium calculations in this chapter, we’ll take the latter approach. The Henderson-Hasselbalch equation requires the concentrations of HCO2? and HCO2H, which can be calculated using the number of millimoles (n) of each and the total volume (VT). Substituting these values into the Henderson-Hasselbalch equation,