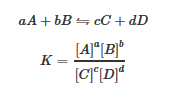Tags | |
UUID | 19f93e3f-f145-11e9-8682-bc764e2038f2 |
Connection Between Ecell, delta G, and K
From UCDavis Chemwiki
|
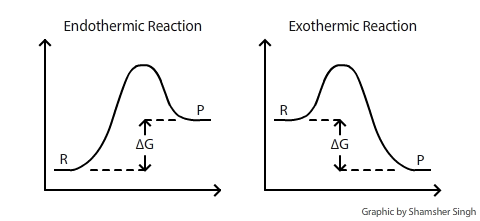
The connection between cell potential, Gibbs free energy and constant equilibrium are directly related in the following multi-part equation: ?Go=?RTlnKeq=?nFEocell ?G: Gibbs Free Energy?G is the change of Gibbs (free) energy for a system and ?G° is the Gibbs energy change for a system under standard conditions (1 atm, 298K). On an energy diagram, ?G can be represented as:
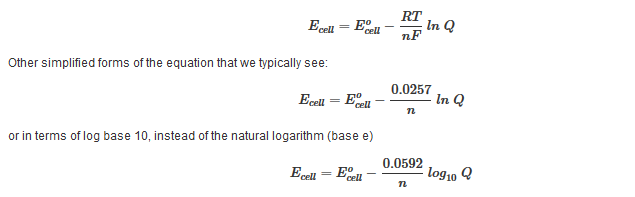
Where ?G is the difference in the energy between reactants and products. In addition ?G is unaffected by external factors that change the kinetics of the reaction. For example if Ea(activation energy) were to decrease in the presence of a catalyst or the kinetic energy of molecules increases due to a rise in temperature, the ?G value would remain the same. E°cell: Standard Cell PotentialE°cell is the electromotive force (also called cell voltage or cell potential) between two half-cells. The greater the E°cell of a reaction the greater the driving force of electrons through the system, the more likely the reaction will proceed (more spontaneous). E°cell is measured in volts (V). The overall voltage of the cell = the half-cell potential of the reduction reaction + the half-cell potential of the oxidation reaction. To simplify, Ecell = Ereduction + Eoxidation or Ecell = Ecathode + Eanode The potential of an oxidation reduction (loss of electron) is the negative of the potential for a reduction potential (gain of electron). Most tables only record the standard reduction half-reactions. In other words, most tables only record the standard reduction potential; so in order to find the standard oxidation potential, simply reverse the sing of the standard reduction potential. *Note: The more positive reduction potential of reduction reactions are more spontaneous. When viewing a cell reduction potential table, the higher the cell is on the table, the higher potential it has as an oxidizing agent. Difference between Ecell and EºcellEº cell is the standard state cell potential, which means that the value was determined under standard states. The standard states include a concentration of 1 Molar (mole per liter) and an atmospheric pressure of 1. Similar to the standard state cell potential, Eºcell, the Ecell is the non-standard state cell potential, which means that it is not determined under a concentration of 1 Molar and pressure of 1 atm. The two are closely related in the sense that the standard cell potential is used to calculate for the cell potential in many cases. Both equations applies when the temperature is 25ºC. Deviations from 25ºC requires the use of the original equation. Essentially, Eº is E at standard conditions
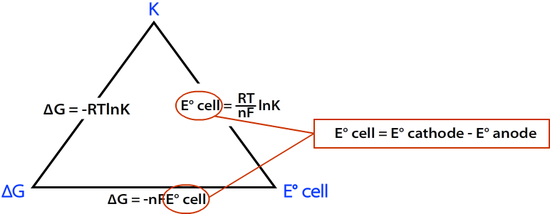
K: The Equilibrium ConstantK is the equilibrium constant of a reaction and is given by the reaction quotient: The Relationship Between The ThreeThe relationship between ?G, K, and E° cell can be represented by the following diagram.
where
E°cell can be calculated using the following formula: E°cell = E° (cathode) – E° (anode) = E°(Reduction) – E°(Oxidation)
|
This Collection is empty
- Comments
- Attachments
- Stats
No comments |