Tags | |
UUID | 19fa51e5-f145-11e9-8682-bc764e2038f2 |
Electrolytic Cells
From UCDavis Chemwiki
|
Voltaic cells are driven by a spontaneous chemical reaction that produces an electric current through an outside circuit. These cells are important because they are the basis for the batteries that fuel modern society. But they are not the only kind of electrochemical cell. The reverse reaction in each case is non-spontaneous and requires electrical energy to occur. IntroductionThe general form of the reaction can be written as:
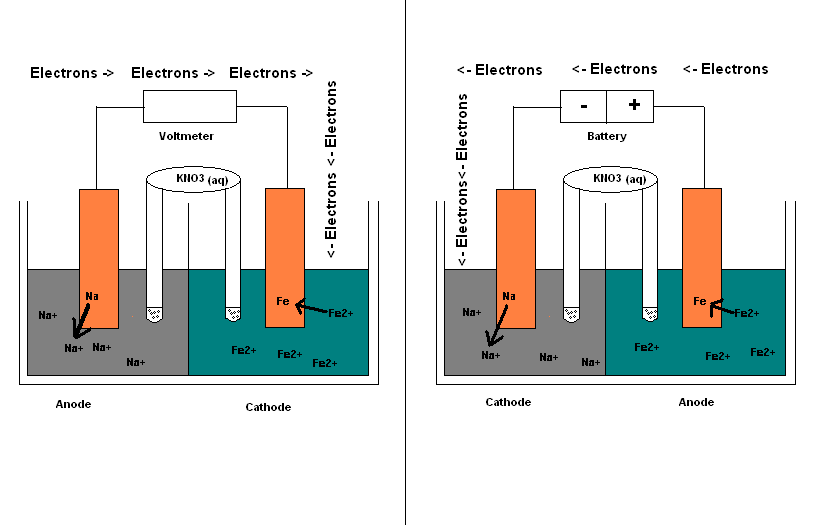
It is possible to construct a cell that does work on a chemical system by driving an electric current through the system. These cells are called electrolytic cells, and operate through electrolysis. Electrolysis is used to drive an oxidation-reduction reaction in a direction in which it does not occur spontaneously by driving an electric current through the system while doing work on the chemical system itself, and therefore is non-spontaneous. Electrolytic cells, like galvanic cells, are composed of two half-cells--one is a reduction half-cell, the other is an oxidation half-cell. The direction of electron flow in electrolytic cells, however, may be reversed from the direction of spontaneous electron flow in galvanic cells, but the definition of both cathode and anode remain the same, where reduction takes place at the cathode and oxidation occurs at the anode. Because the directions of both half-reactions have been reversed, the sign, but not the magnitude, of the cell potential has been reversed. Similarities and Differences Galvanic and Electrolytic Cell:
Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. However, there are also striking differences between the two cells. The main differences are outlined below: Differences between a Galvanic cell and an Electrolytic cell
Electrolytic Cell
If molten NaCl (l) is placed into the container and inert electrodes of C(s) are inserted , attached to the + and - terminals of a battery, an electrolytic reaction will occur.
Predicting Electrolysis ReactionThere are four factors that determine whether or not electrolysis will take place even if the external voltage exceeds the calculated amount: 1) An overpotential or voltage excess is sometimes needed to overcome interactions at the electrode surface. This case happens more frequently with gases. Ex1) H2 (g) : 1.5 V overpotential Pt (s) : 0 V overpotential 2)There might be more than one electrode reaction that occurs meaning that there may be more than one half-reaction leaving two or more possibilities for the cell reaction. 3) The reactants may be in nonstandard conditions which means that the voltage for the half cells may be less or more than the standard condition amount Ex1) Concentration of chloride ion = 5.5M not the unit activity of 1M. This means that the reduction of chloride = 1.31V not 1.36V Ex2) The standard condition is to have a pH of 4 in the anode half cell but sometimes during nonstandard states, the pH may be higher or lower changing the voltage. 4) An inert electrode’s ability to electrolysis depend on the reactants in the electrolyte solution while an active electrode can run on its own to perform the oxidation or reduction half reaction. If all four of these factors are accounted for, we can successfully predict electrode half reactions and overall reactions in electrolysis. Example) Predict the electrode reactions and the overall reaction when the anode is made of (a) copper and (b) platinum. Quantitative Aspects of ElectrolysisMichael Faraday discovered in 1833 that there is always a simple relationship between the amount of substance produced or consumed at an electrode during electrolysis and the quantity of electrical charge Q which passes through the cell. For example, the half-equation Ag+ + e– ? Ag tells us that when 1 mol Ag+ is plated out as 1 mol Ag, 1 mol e– must be supplied from the cathode. Since the negative charge on a single electron is known to be 1.6022 × 10–19 C, we can multiply by the Avogadro constant to obtain the charge per mole of electrons. This quantity is called the Faraday Constant, symbol F: F = 1.6022 × 10–19 C × 6.0221 × 1023 mol–1 = 9.649 × 104 C mol–1 Thus in the case of Eq. (1), 96 490 C would have to pass through the cathode in order to deposit 1 mol Ag. For any electrolysis the electrical charge Q passing through an electrode is related to the amount of electrons ne– by
Thus F serves as a conversion factor between ne– and Q. Often the electrical current rather than the quantity of electrical charge is measured in an electrolysis experiment. Since a coulomb is defined as the quantity of charge which passes a fixed point in an electrical circuit when a current of one ampere flows for one second, the charge in coulombs can be calculated by multiplying the measured current (in amperes) by the time (in seconds) during which it flows: Q = It In this equation I represents current and t represents time. If you remember that coulomb = 1 ampere × 1 second 1 C = 1 A s you can adjust the time units to obtain the correct result. Now that we can predict the electrode half-reactions and overall reactions in electrolysis, it is also important to be able to calculate the quantities of reactants consumed and the products produced. For these calculations we will be using the Faraday constant: 1 mol of electron = 96,485 C charge (C) = current (C/s) x time (s) (C/s) = 1 coulomb of charge per second = 1 ampere (A) Simple conversion for any type of problem:
Example) The electrolysis of dissolved Bromine sample can be used to determine the amount of Bromine content in sample. At the cathode, the reduction half reaction is Br2+(aq) + 2 e- -> 2 Br-. What mass of Bromine can be deposited in 3.00 hours by a current of 1.18 A? Sol) 3.00 hours x 60 min/hour x 60 sec/1 min x 1.18 C(A) / 1 sec x 1 mol e-/96,485 C = 0.132 mol e- |
||||||||||||||||||||||||||||||||||||||||||||||||||||
This Collection is empty
- Comments
- Attachments
- Stats
No comments |