Tags | |
UUID | 19ff446f-f145-11e9-8682-bc764e2038f2 |
Answers to Chapter 20 End-of-Chapter Problems
|
1. Anode 2. It is important in calculating half-cell potentials because it serves as a reference. Without this electrode, there would be no basis to calculate values of cell potentials. 3. The left is the anode and the right is the cathode. 4. It is important to use an inert electrode in this situation because it will not react or participate in the reaction in the cell, just provide a surface area for the reaction to occur. 5. 0 volts. 6. a. i) Ba2+(aq) + 2e- ? Ba(s) Eo = -2.92 V Anode b.i) Ba2+(aq) | Ba(s) || Cu(s) | Cu2+(aq) b. ii) Al(s) | Al3+(aq) || Sn2+(aq) | Sn(s) c. i) Eocell = 0.34 - (-2.92) = 3.26 V c. ii) Eocell = -0.137 - (-1.66) = 1.523 V 7. oxidation: Sn(s) ? Sn2+(aq) + 2e-(aq)
Eocell= 2.71V= +0.401V - Eoox = -2.31V 13. Cl- chlorine H+ hydrogen Cl- chlorine Cu2+ copper I- iodine H+ Hhydrogen 14. 12 mol e– is required to plate 2 mol Cr, giving us a stoichiometric ratio S(e–/Cr). Then the Faraday constant can be used to find the quantity of charge. nCr Q = 1.386 mol Cr × 15. The product of current and time gives us the quantity of electricity, Q. Knowing this we easily calculate the amount of electrons, ne–. From the first half-equation we can then find the amount of peroxydisulfuric acid, and the second leads to nH2O2 and finally to mH2O2.
= 05666 16. 0.259 mol e- 17. d 18.d 19. b 20. d 21. Write the half-reactions for each process. Zn(s) ? Zn2+(aq) + 2 e- Cu2+(aq) + 2 e- ? Cu(s) Look up the standard potentials for the reduction half-reaction. Eoreduction of Cu2+ = + 0.339 V Eoreduction of Zn2+ = - 0.762 V Determine the overall standard cell potential. Eocell = + 1.101 V 22. +1139.68 kJ 23. 6.4 x 1025 24.+0.195 V |
Calculators and Collections
- Comments
- Attachments
- Stats
No comments |

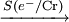 ne–
ne– 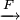 Q
Q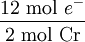 ×
× 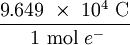 = 8.024 × 105 C
= 8.024 × 105 C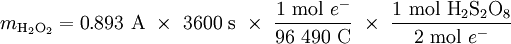
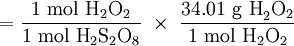
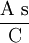 × g H2O2 = 0.5666 g H2O2
× g H2O2 = 0.5666 g H2O2