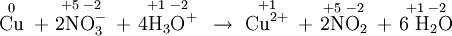Tags | |
UUID | 1a0113cd-f145-11e9-8682-bc764e2038f2 |
Oxidation Numbers Review
From ChemPRIME
|
Redox reactions may involve proton transfers and other bond-breaking and bond-making processes, as well as electron transfers, and therefore the equations involved are much more difficult to deal with than those describing acid-base reactions. In order to be able to recognize redox reactions, we need a method for keeping a careful account of all the electrons. This is done by assigning oxidation numbers to each atom before and after the reaction. For example, in NO3– the nitrogen is assigned an oxidation number of +5 and each oxygen an oxidation number of –2. This arbitrary assignment corresponds to the nitrogen’s having lost its original five valence electrons to the electronegative oxygens. In NO2, on the other hand, the nitrogen has an oxidation number of + 4 and may be thought of as having one valence electron for itself, that is, one more electron than it had in NO3–. This arbitrarily assigned gain of one electron corresponds to reduction of the nitrogen atom on going from NO3– to NO2. As a general rule, reduction corresponds to a lowering of the oxidation number of some atom. Oxidation corresponds to increasing the oxidation number of some atom. Applying the oxidation number rules to the following equation, we have
Since the oxidation number of copper increased from 0 to +2, we say that copper was oxidized and lost two negatively charged electrons. The oxidation number of nitrogen went down from 5 to 4, and so the nitrogen (or nitrate ion) was reduced. Each nitrogen gained one electron, so 2e– were needed for the 2 NO3–. The nitrogen was reduced by electrons donated by copper, and so copper was the reducing agent. Copper was oxidized because its electrons were accepted by an oxidizing agent, nitrogen (or nitrate ion). Although they are useful and necessary for recognizing redox reactions, oxidation numbers are a highly artificial device. The nitrogen atom in NO3– does not really have a +5 charge which can be reduced to +4 in NO2. Instead, there are covalent bonds and electron-pair sharing between nitrogen and oxygen in both species, and nitrogen has certainly not lost its valence electrons entirely to oxygen. Even though this may (and indeed should) make you suspicious of the validity of oxidation numbers, they are undoubtedly a useful tool for spotting electron-transfer processes. So long as they are used for that purpose only, and not taken to mean that atoms in covalent species actually have the large charges oxidation numbers often imply, their use is quite valid. EXAMPLE 2 Identify the redox reactions and the reducing and oxidizing agents from the following: a) 2MnO4– + 5SO2 + 6H2O ? 5SO42– + 2Mn2+ + 4H3O+ b) NH4+ + PO43– ? NH3 + PO42– c) HClO + H2S ? H3O+ + Cl– + S |
Calculators and Collections
- Comments
- Attachments
- Stats
No comments |