Delocalization is a characteristic of the molecular orbital theory concerning the structure of atoms. Rather than the lone pair of electrons contained in specific bonds (as in the valence-bond theory), the MO (molecular-orbital theory) theorizes that electrons exist in orbitals that are spread over the entire molecule. The MO theory explains molecules such as ozone and benzene, which cannot be drawn satisfactorily with one Lewis structure, and are therefore described as resonance hybrids. Molecular orbitals solve this issue through the concept of delocalized electrons.
When orbitals overlap, many of the orbitals are in hybrid states. Like delocalization, hybridization occurs to promote symmetry and stability. Delocalized electrons are often found in covalently bonded molecules that alternate single and multiple (usually double) bonds. Also, they frequently occur in aromatic systems and in mesoionic systems which means that they cannot exist in merely one resonance structure (they can and do exist in many resonance structures).
Delocalization matters for several reasons. One, spreading electron densities over a great area creates greater stability for the molecule and spreading electrons creates charge distribution. Therefore, chemical reactions expected because of a hypothesized configuration may not occur, a stable product is formed more readily in a reaction. Delocalization of electrons also paves the way for conductivity while in spectroscopy, wavelength absorption is also based on resonance stability and delocalization of electrons.
The MO treatment can also be used to interpret the spectrum of ozone. Ozone in the earth’s stratosphere absorbs much solar ultraviolet radiation which would otherwise cause damage to the biosphere. This absorption is due to a band centered around 255 nm which corresponds to excitation of an electron to the unfilled antibonding pi orbital.
Another important molecule to which MO theory can be applied usefully is benzene. Benzene can be represented by the resonance hybrid
-
-
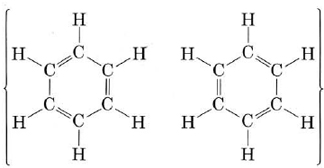
This indicates that all C—C bonds are equivalent and intermediate between a single and a double bond. As in the case of ozone, we can treat the sigma bonds of benzene in valence-bond terms. We are only able to deal with pi bonding by using the molecular-orbital, method. The sigma bonding framework is
-
-
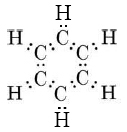
This involves 24 electrons in bonds formed by overlap of sp2 hybrid orbitals on the carbon atoms with 1s orbitals on each hydrogen or with other sp2 hybrids on other carbons. This gives a planar framework and leaves six p orbitals (one on each carbon) which are perpendicular to the molecular plane and can overlap sideways to form six pi molecular orbitals. The MO theory predicts that the six 2p orbitals of the C atoms overlap and mix to form three pi-bonding and three pi-antibonding molecular orbitals. The six pi electrons occupy the three lowest energy MOs, the bonding MOs. Thus, they are distributed throughout the molecule as a whole as seen in Figure 7. This results in identical character for all carbon-carbon bonds in benzene.
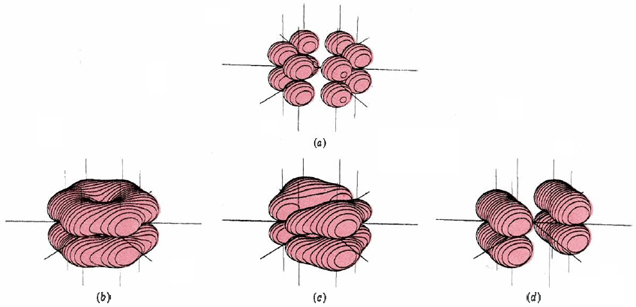 Figure 8. The pi molecular orbitals in benzene. When the six p orbitals shown in (a) are combined, they can form a total of six molecular orbitals. Only three of these are occupied, and they are shown in (b), (c), and (d). The net effect of the three filled orbitals (b), (c), and (d) is a double hexagonal ring similar to (b) but slightly larger. This contains six electrons, giving three bonds evenly distributed between all six carbon atoms.
Figure 8. The pi molecular orbitals in benzene. When the six p orbitals shown in (a) are combined, they can form a total of six molecular orbitals. Only three of these are occupied, and they are shown in (b), (c), and (d). The net effect of the three filled orbitals (b), (c), and (d) is a double hexagonal ring similar to (b) but slightly larger. This contains six electrons, giving three bonds evenly distributed between all six carbon atoms.
For a description of Figure 8, click on the figure.





