Tags | |
UUID | 1a08aa92-f145-11e9-8682-bc764e2038f2 |
Example 5
| YouTube Video
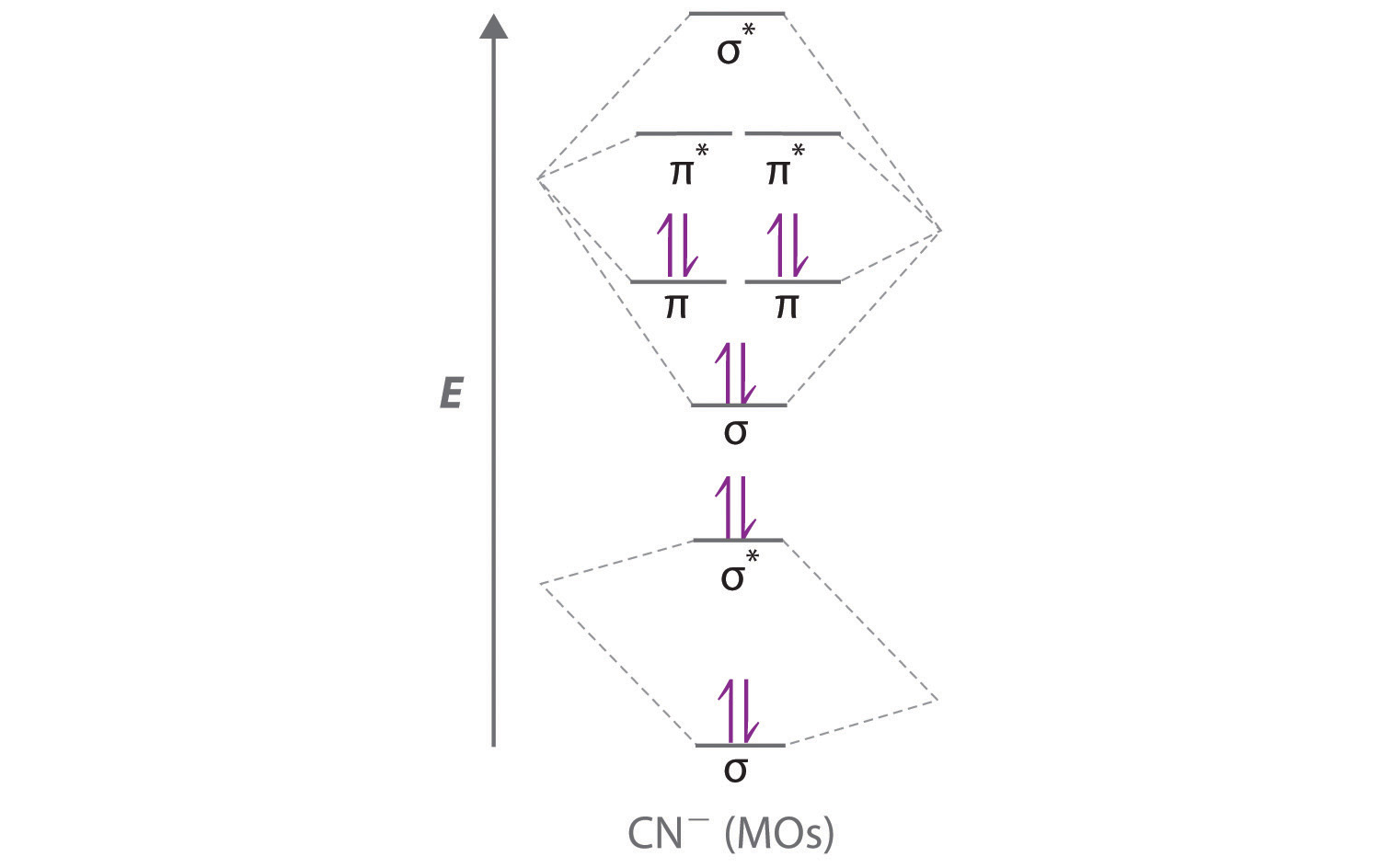
A The CN? ion has a total of 10 valence electrons: 4 from C, 5 from N, and 1 for the ?1 charge. Placing these electrons in an energy-level diagram like Figure 6 "Molecular Orbital Energy-Level Diagram for a Heteronuclear Diatomic Molecule AB, Where ?" fills the five lowest-energy orbitals, as shown here:
|
Calculators and Collections
- Comments
- Attachments
- Stats
No comments |
This site uses cookies to give you the best, most relevant experience. By continuing to browse the site you are agreeing to our use of cookies.