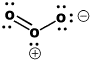Tags | |
UUID | 1a093952-f145-11e9-8682-bc764e2038f2 |
End of Ch Q8 Answer
Delocalized Bonding: Conjugation in OzoneOzone is a fairly simple molecule, with only three atoms. However, in order to focus on one aspect of ozone's structure, we will use a hybrid approximation in order to simplify the picture.
The Lewis structure of ozone is somewhat unsatisfactory. That's because the true structure of ozone can't be drawn easily using Lewis conventions. Ozone is an angular structure in which both oxygen-oxygen bonds are about 1.278 Angstroms long. However, the Lewis structure of ozone does not reflect that reality. In the Lewis structure, one pair of oxygens is double-bonded and the other is single-bonded. If this were true, there would be two different bond lengths in ozone. One bond would be about 1.49 Angstroms long, like the O-O bond in peroxide. The other bond would be about 1.208 Angstroms long, like the O=O bond in dioxygen. The bond in ozone looks pretty close to a double bond, doesn't it? It is just a little longer, however.
In Lewis structures, we fix this discrepancy by drawing two resonance structures for ozone. In one structure, the double bond is between one pair of oxygens. In the other structure, the double bond is between the other pair. The resonance structures imply that the real structure is somewhere in between the two that are shown. Roughly speaking, there should be one-and-a-half bonds between the neighbouring oxygens. Nevertheless, Lewis structures have trouble illustrating the nature of the double bond in ozone, which seems to be both there and not there at the same time. We could get another look at bonding in ozone using a molecular orbital approach. We are basically concerned with one question: what is the nature of the double bond? We already know about double bonds. We have seen them in compounds like nitrogen. A second bond is generally made through a pi bonding interaction. Therefore, we are only going to worry about the orbitals that will form pi bonds.
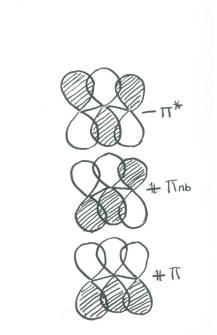
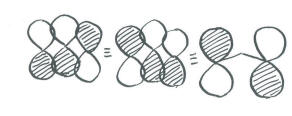
We have three orbitals to combine. Therefore, there will be three combinations.
Populating these orbitals, and getting an exact energy, is not possible given the huge approximations we have made. However, in focusing on the pi bonding, we see something that we can't see in Lewis terms. There really is a pi bond that stretches the entire length of the ozone molecule. This is the lowest energy combination, with a wavelength steretching over twice the length of the molecule.
Because it is low in energy, the extended pi bond is pretty certain to be populated by electrons, and it will make some contribution to the structure of ozone. How much impact it has would depend on the population of the other combinations, which we can't predict without a more careful approach. |
Calculators and Collections
- Comments
- Attachments
- Stats
No comments |