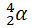Tags | |
UUID | 1a0aa995-f145-11e9-8682-bc764e2038f2 |
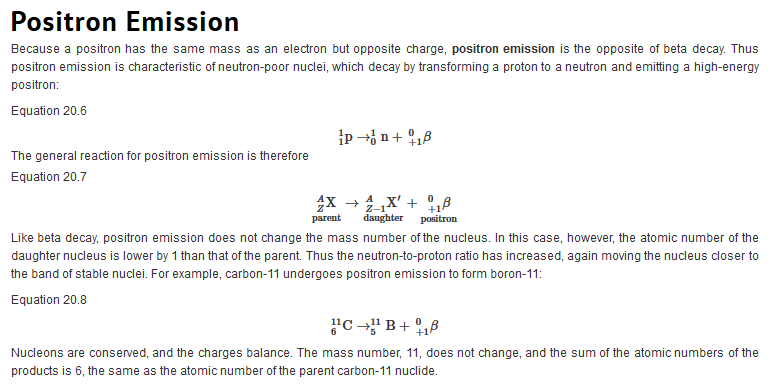
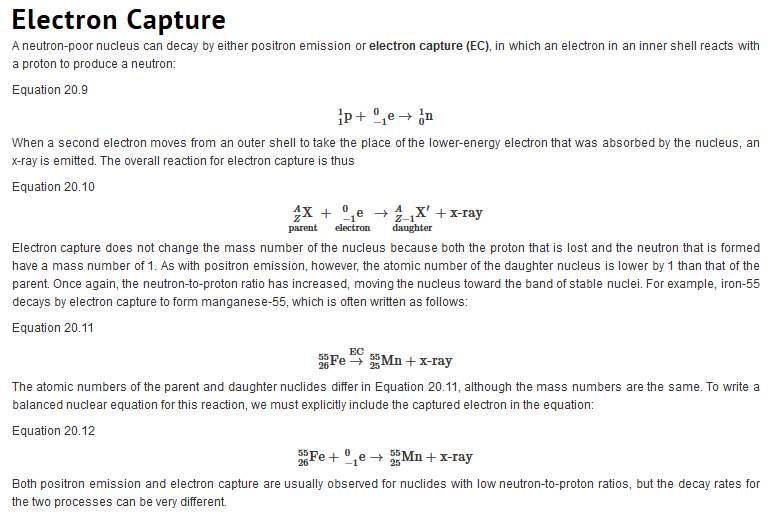
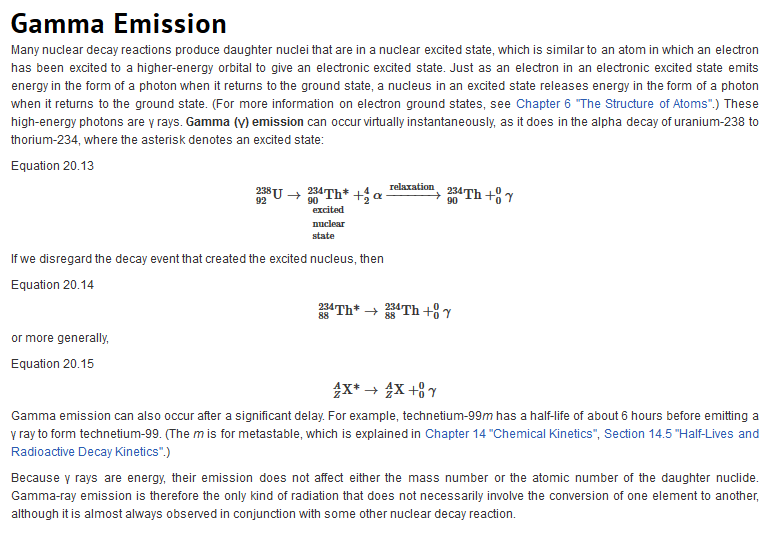
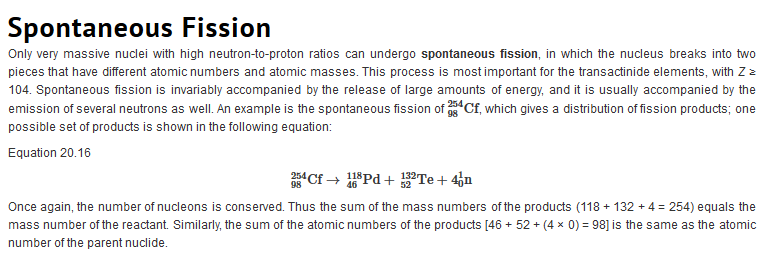
Types of Radiation
From UCDavis Chemwiki
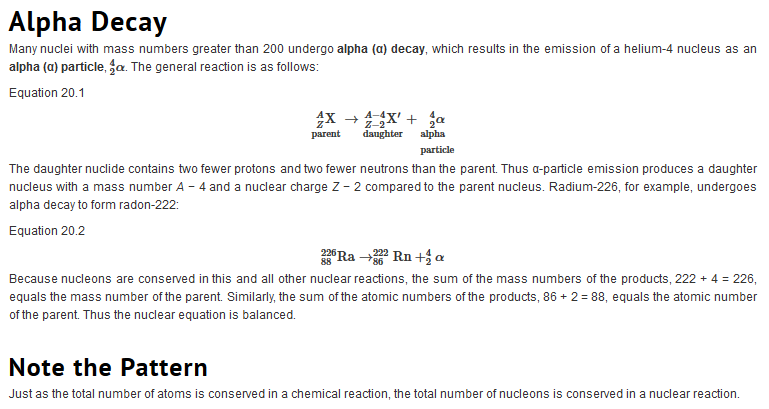
| Just as we use the number and type of atoms present to balance a chemical equation, we can use the number and type of nucleons present to write a balanced nuclear equation for a nuclear decay reaction. This procedure also allows us to predict the identity of either the parent or the daughter nucleus if the identity of only one is known. Regardless of the mode of decay, the total number of nucleons is conserved in all nuclear reactions.
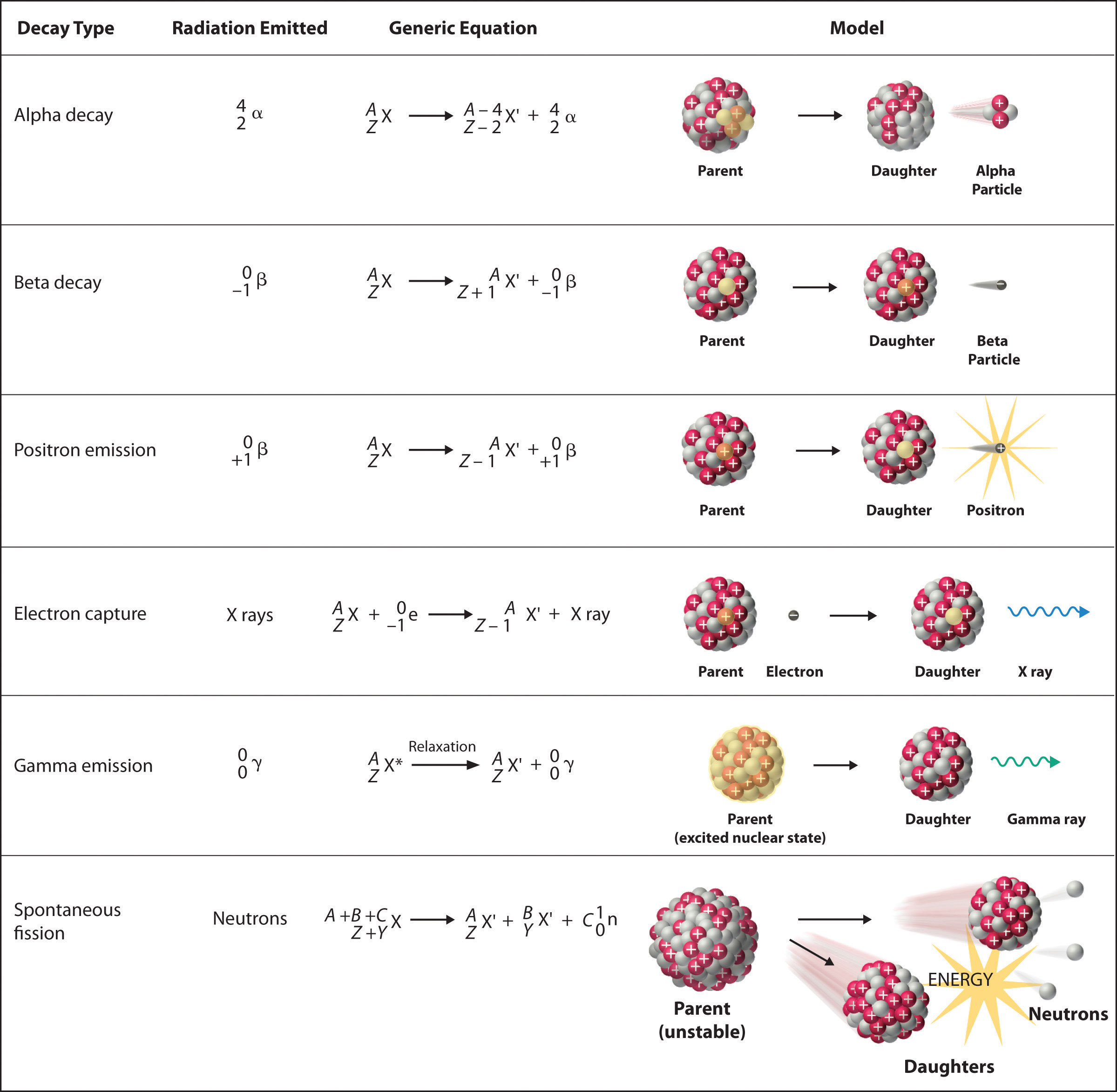
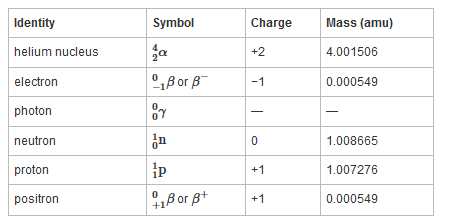
To describe nuclear decay reactions, chemists have extended the notation for nuclides to include radioactive emissions. Table 26.1 "Nuclear Decay Emissions and Their Symbols" lists the name and symbol for each type of emitted radiation. Table 26.1 Nuclear Decay Emissions and Their Symbols
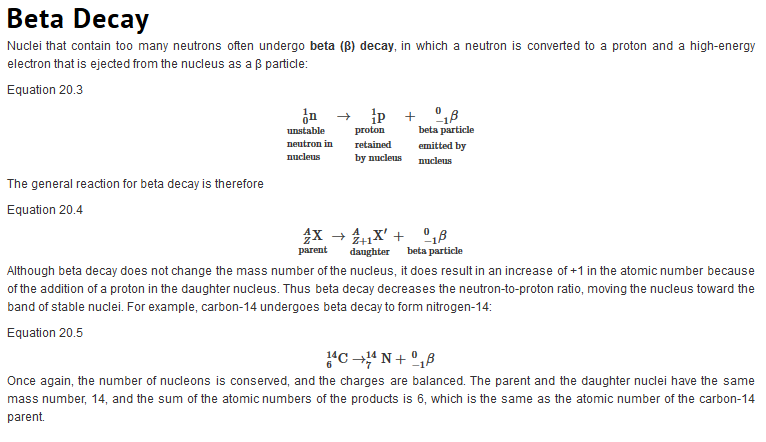
Like the notation used to indicate isotopes, the upper left superscript in the symbol for a particle gives the mass number, which is the total number of protons and neutrons. For a proton or a neutron, A = 1. Because neither an electron nor a positron contains protons or neutrons, its mass number is 0. The numbers should not be taken literally, however, as meaning that these particles have zero mass; ejection of a beta particle (an electron) simply has a negligible effect on the mass of a nucleus. Similarly, the lower left subscript gives the charge of the particle. Because protons carry a positive charge, Z = +1 for a proton. In contrast, a neutron contains no protons and is electrically neutral, so Z = 0. In the case of an electron, Z = ?1, and for a positron, Z = +1. Because ? rays are high-energy photons, both A and Z are 0. In some cases, two different symbols are used for particles that are identical but produced in different ways. For example, the symbol
which is usually simplified to e?, represents a free electron or an electron associated with an atom, whereas the symbol
which is often simplified to ??, denotes an electron that originates from within the nucleus, which is a ? particle. Similarly,
Figure 20.4 Common Modes of Nuclear Decay
|
This Collection is empty
- Comments
- Attachments
- Stats
No comments |

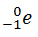
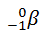
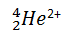 refers to the nucleus of a helium atom, and
refers to the nucleus of a helium atom, and