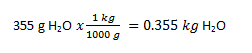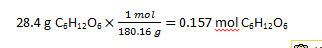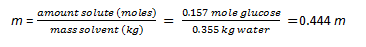Tags | |
UUID | 19a81850-f145-11e9-8682-bc764e2038f2 |
Chapter 11 End-of-Chapter Answers
(c) Pentane, C5H12, in CCl4 Yes (Polar and polar) (d) methanol, CH3OH, in water Yes (Polar and polar) (e) Vegetable oil in mineral oil Yes (non-polar and non-polar) 5. (a) 6. a) hexane, toluene, or CCl4 (dispersion forces); b) same as a; c) water, acetone, methanol, ethanol (dispersion forces, ion-dipole); d) same as a and b 7. When the temperature of the solution (water as solvent and oxygen gas as the solute) is raised the solubility of the oxygen in water is lowered so the gas bubbles out of solution. The result is much less oxygen in the water solution. When the fish is put in this solution with to little oxygen, it can not survive. 8. C = kP = 3.7 x 10-4 M/atm · 1.0 atm = 3.7x10-4 M 9. First convert the mass of water to kg. Next you will convert the grams of glucose to moles.
Calculate the molality. 10. Solution P pure H2O = 23.8 mm Hg To solve for the mole fraction, you must first convert the 2 L of water into moles: 1 L = 1000 mL = 1000 g Knowing this, you can convert the mass of water (2000 g) into moles: 2000 g / 18.02 g (molar mass of water) = 110.9 moles H2O Solve for the mole fraction, xH2O: xH2O = moles H2O / total moles Finally, apply Raoult's Law PKool-Aid = xH2O Ppure H2O = (.979)(23.8 mm Hg) = 23.3 mm Hg 11. Solution If xbenzene = 0.6, than xtoluene = 0.4 because 1 - 0.6 = 0.4. Now that we know the mole fractions and vapor pressures, this problem is a cinch. Pbenzene = xbenzenePbenzene = (0.6)(95.1 mm Hg) = 57.1 mm Hg The total vapor pressure is simply the sum of the partial pressures: Ptotal = Pbenzene + Ptuolene = 57.1 mm Hg + 11.4 mm Hg = 68.5 mm Hg 12. The KbH2O= 0.512°C/m so ?Tb = (0.0512°C/m)(1.25m) = 0.640°C The solution would boil at a temperature that is 0.640°C higher than pure water (boiling point elevation). The normal boiling point of pure water is 100°C so we would predict this solution to boil at 100°C+0.640°C = 100.640°C. 13. 25°C + 273.15 = 298.15K 500.0 mL x (1L/1000mL) = 0.5000 L 8.92 g KBr (1 mol KBr/199.01 g KBr) = 0.0750 mol KBr Calculate the Molarity of the solution: M = 0.0750 mol KBr/ 0.5000 L = 0.150 M KBr ? = MRT = (0.150 M) (0.08206 L*atm/mol*K) (298K) = 3.67 i = actual osmotic pressure/osmotic pressure that would be observed if no ionic dissociation occured = 6.97/3.67 = 1.899 We expect i to be 2 if the KBr completely dissociates so this value seems reasonable. 14. (c) |
This Collection is empty
- Comments
- Attachments
- Stats
No comments |