Tags | |
UUID | 19babe13-f145-11e9-8682-bc764e2038f2 |
Example 10
|
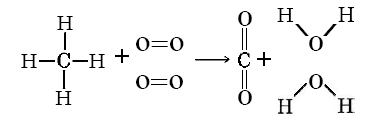
See the calculation on this YouTube video Solution It is best to sketch the molecules and their bonds in order to make sure that none are missed. Thus ?H° = ?D (bond broken) – ?D (bond formed) = 4 DC?H + 2DO=O – 2DC=O – 4DO?H = (4 × 416 + 2 × 498 – 2 × 803 – 4 × 467) kJ mol–1 = – 814 kJ mol–1 |
This Collection is empty
- Comments
- Attachments
- Stats
No comments |
This site uses cookies to give you the best, most relevant experience. By continuing to browse the site you are agreeing to our use of cookies.