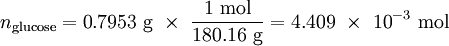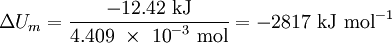Tags | |
UUID | 19bb31eb-f145-11e9-8682-bc764e2038f2 |
Example 11
|
See the calculation on this YouTube video Solution The heat energy absorbed by the calorimeter in increasing its temperature by 1.841 K is given by q = C ?T = 6.745 kJ K–1 × 1.841 K = 12.42 kJ Since this heat energy was released by the reaction system, we must regard it as negative. Accordingly, qV = –12.42 kJ = ?U We need now only to calculate the change in internal energy per mole, that is, ?Um. Now
Thus |
This Collection is empty
- Comments
- Attachments
- Stats
No comments |
This site uses cookies to give you the best, most relevant experience. By continuing to browse the site you are agreeing to our use of cookies.