Tags | |
UUID | 19dc9010-f145-11e9-8682-bc764e2038f2 |
Example 8
Solution
a) The Lewis diagram
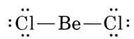
shows that Be is electron deficient. Therefore BeCl2(g) is a Lewis acid. Because of the lone pairs on the Cl atoms, BeCl2 can also act as a Lewis base, but Cl is rather electronegative and reluctant to donate electrons, so the Lewis base strength of BeCl2 is less than the Lewis acid strength.
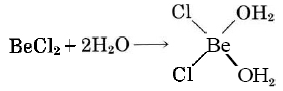
b) There are lone pairs on O in CH3OH, and so it can serve as a Lewis base.
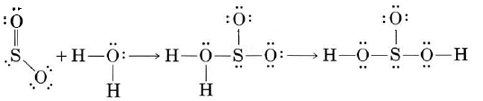
c) The S atom in SO2 can accept an extra pair of electrons, and so SO2 is a Lewis acid. The O atoms have lone pairs but are only weakly basic for the same reason as the Cl atoms in part (a).
d) Although there are lone pairs on the F atoms, the high electronegativity of F prevents them from being donated to form coordinate covalent bonds. Consequently CF4 has essentially no Lewis-base character.
This Collection is empty
- Comments
- Attachments
- Stats
No comments |
