Tags | |
UUID | 19e09755-f145-11e9-8682-bc764e2038f2 |
Example 3
Click here to see the calculation on Video
Solution The known volume and concentration allow us to calculate the amount of NaOH(aq) which reacted with all the vitamin C. Using the stoichiometric ratio
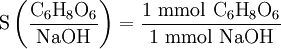
we can obtain the amount of C6H8O6. The molar mass converts that amount to a mass which can be compared with the label. Schematically
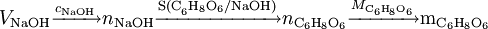
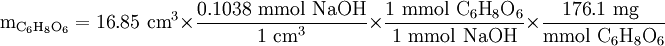
= 308.0 mg
Note that the molar mass of C6H8O6
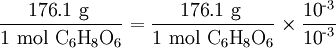
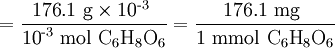
can be expressed in milligrams per millimole as well as in grams per mole.
This Collection is empty
- Comments
- Attachments
- Stats
No comments |
This site uses cookies to give you the best, most relevant experience. By continuing to browse the site you are agreeing to our use of cookies.
