Tags | |
UUID | 19f0520d-f145-11e9-8682-bc764e2038f2 |
Henderson Hasselbalch Equation
From UCDavis Chemwiki
The Henderson-Hasselbalch formula allows us one method to approximate the pH of a buffer solution. The basic equation is as follows:
| vCalc Companion Formulas | |
| vCalc Formulary | CHM1 18 Henderson H Equation |
| pH=pKa+(log([A-])[HA]) | Henderson Hasselbalch Equation |
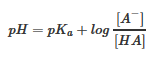
Motivation
We have straightforward caluclations for strong acids and bases, but the computations behind buffers are rather complex and time consuming. By using the fact that weak acids and bases barely ionize, allowing us to approximate the pH of buffer solutions using initial concentrations. Though the approximation has a few restrictions, it simplifies a lenghty calculation into a simple equation derived from K.
Brief History
- Lawrence Joseph Henderson (1878-1942) was a talented biochemist, among many other titles, who spent most of his career at Harvard. He was responsible for developing the components of the equation after studying equilibrium reactions that took place within blood as a result of respiration (specializing in "fatigue"). His equation was incomplete without a solid calculations going into it.M
- Karl Albert Hasselbalch (1874-1962) was a chemist who studied pH closely. He also studied blood and reactions that took place with oxygen, to put in the simplest of terms. He eventually modified Henderson's equation by putting mathematical logs into it creating a solid relationship.J
Requirements
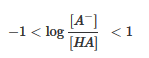
- The molarity of the buffer(s) should be 100x that of the acid ionization constant, or Ka.
- This equation will give poor or inaccurate results if there are strong acids or bases. pKa values between 5 and 9 will give good approximations, but when we are out of this range there is a strong chance that the pH value will be incorrect.J In other words near the end or the beginning of a titration where the "relative concentrations of the acid or base differ substantially[,]... approximate calculations break down."J
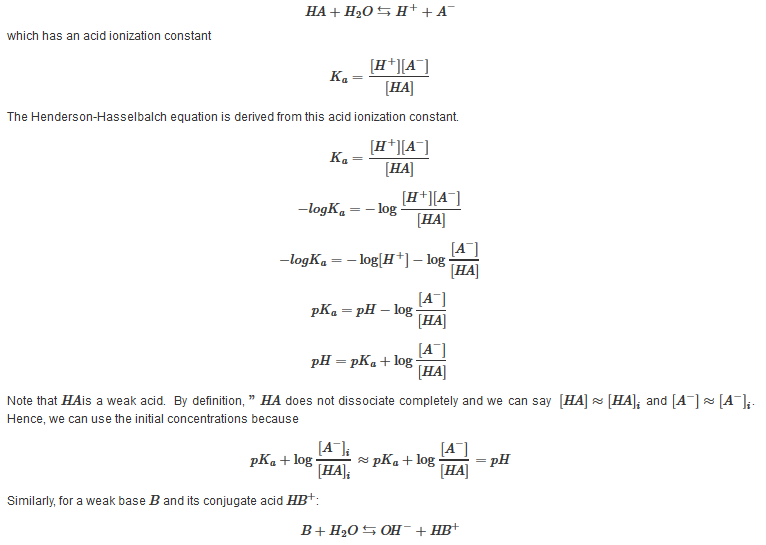
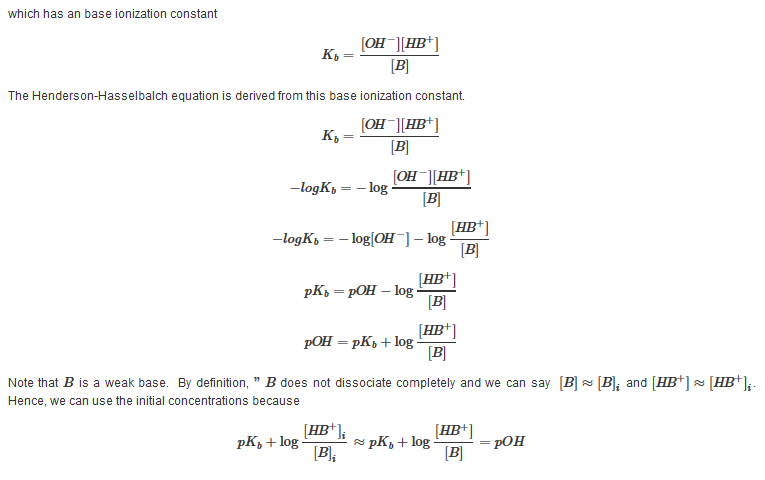
Derivation
For a weak acid HA and its conjugate base A?:
This Collection is empty
- Comments
- Attachments
- Stats
No comments |
This site uses cookies to give you the best, most relevant experience. By continuing to browse the site you are agreeing to our use of cookies.
