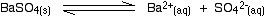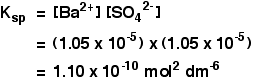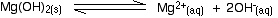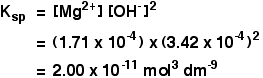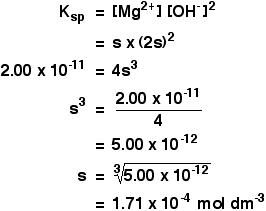Tags | |
UUID | 19f47b1a-f145-11e9-8682-bc764e2038f2 |
Calculations Involving Solubility Products
From UCDavis Chemwiki
|
Calculating solubility products from solubilities It is assumed that you are given the solubility of an ionic compound in mol dm-3. If it was in g dm-3, or any other concentration units, you would first have to convert it into mol dm-3.
Example 3 The solubility of barium sulphate at 298 K is 1.05 x 10-5 mol dm-3. Calculate the solubility product. The equilibrium is: Notice that each mole of barium sulphate dissolves to give 1 mole of barium ions and 1 mole of sulphate ions in solution. That means that:
[Ba2+] = 1.05 x 10-5 mol dm-3
[SO42-] = 1.05 x 10-5 mol dm-3
All you need to do now is to put these values into the solubility product expression, and do the simple sum.
Don't forget to work the units out.
Example 4 These calculations are very simple if you have a compound in which the numbers of positive and negative ions are 1 : 1. This next example shows you how to cope if the ratio is different. The solubility of magnesium hydroxide at 298 K is 1.71 x 10-4 mol dm-3. Calculate the solubility product. The equilibrium is: For every mole of magnesium hydroxide that dissolves, you will get one mole of magnesium ions, but twice that number of hydroxide ions. So the concentration of the dissolved magnesium ions is the same as the dissolved magnesium hydroxide: The concentration of dissolved hydroxide ions is twice that: [OH-] = 2 x 1.71 x 10-4 = 3.42 x 10-4 mol dm-3
Now put these numbers into the solubility product expression and do the sum. Calculating solubilities from solubility products Reversing the sums we have been doing isn't difficult as long as you know how to start. We will take the magnesium hydroxide example as above, but this time start from the solubility product and work back to the solubility. If the solubility product of magnesium hydroxide is 2.00 x 10-11 mol3 dm-9 at 298 K, calculate its solubility in mol dm-3 at that temperature. The trick this time is to give the unknown solubility a symbol like x or s. I'm going to choose s, because an x looks too much like a multiplication sign. If the concentration of dissolved magnesium hydroxide is s mol dm-3, then:
[Mg2+] = s mol dm-3
[OH-] = 2s mol dm-3
Put these values into the solubility product expression, and do the sum.
|
This Collection is empty
- Comments
- Attachments
- Stats
No comments |