Tags | |
UUID | 19fbc574-f145-11e9-8682-bc764e2038f2 |
Introduction
From UCDavis Chemwiki
| As the name suggests, electrochemistry is the study of changes that cause electrons to move. This movement of electrons is called electricity. In electrochemistry, electricity can be generated by movements of electrons from one element to another in a reaction known as a redox reaction or oxidation-reduction reaction.
A redox reaction is a reaction which involves a change in oxidation state of one or more elements. When a substance loses its electron, its oxidation state increases, thus it is oxidized. When a substance gains an electron, its oxidation state decreases thus being reduced. For example, for the redox reaction: H2+F2?2HFRewrite it as Oxidation: H2?2H++2e? Reduction: F2+2e??2F? Overall Reaction : H2+F2?2H++2F? Oxidation reaction lose electrons thus they are being oxidized, while Reduction is gaining electrons thus they are being reduced. In this case, H2 is being oxidized, while F2 is being reduced. OIL RIG Oxidation Is Losing electrons Reduction Is Gaining electrons or LEO the lion goes GER Lose Electrons Oxidation, Gain Electrons Reduction. For a full description of RedOx reactions see Chapter 11. Using chemical reactions to produce electricity is now a priority for many researchers. Being able to adequately use chemical reactions as a source of power would greatly help our environmental pollution problems. In this section of electrochemistry, we will be learning how to use chemical reactions to produce this clean electricity and even use electricity to generate chemical reactions. In order to induce a flow of electric charges, we place a strip of metal (the electrode) in a solution containing the same metal, which is in aqueous state. The combination of an electrode and its solution is called a half cell. Within the half cell, metals ions from the solution could gain electrons from the electrode and become metal atoms;or the metal atoms from the electrode could lose electrons and become metals ions in the solution. Introduction to CellsWe use two different half cells to measure how readily electrons can flow from one electrode to another, and the device used for measurement is called a voltmeter. The voltmeter measures the cell potential, denoted by Ecell, (in units of Volts, 1V=1J/C), which is the potential difference between two half cells. The salt bridge allows the ions to flow from one half cell to another but prevents the flow of solutions.
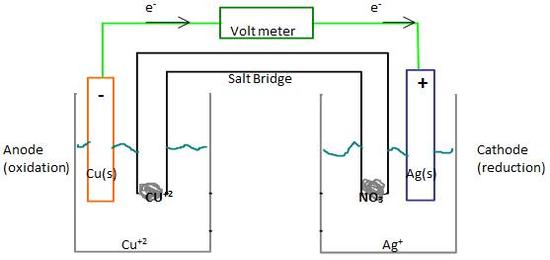
As indicated in the diagram, the anode is the electrode whre oxidation occurs; Cu loses two electrons to form Cu+2. The cathode is the electrode where reduction occurs; Ag+ (aq) gains electron to become Ag(s). As a convenient substitution for the drawing, we use a cell diagram to show the parts of an electrochemical cell. For example above, the cell diagram is : Zn(s) | Zn2+ (aq) || Cu2+ (aq)| Cu(s) oxidation- (half-cell) (salt bridge) (half-cell)-reduction Where we place the anode on the left and cathode on the right, "|" represents the boundary between the two phases, and "||" represents the salt bridge. There are two types of electrochemical cells: A Galvanic Cell (aka Voltaic Cell) induces a spontaneous redox reaction to create a flow of electrical charges, or electricity. Non-rechargeable batteries are examples of Galvanic cells.
An Electrolytic cell is one kind of battery that requires an outside electrical source to drive the non-spontaneous redox reaction. Rechargeable batteries act as Electrolytic cells when they are being recharged.
Both Galvanic and Electrolytic cells contain:
Basic Terminology
|
This Collection is empty
- Comments
- Attachments
- Stats
No comments |