The Cell Potential
From UCDavis Chemiwiki
|
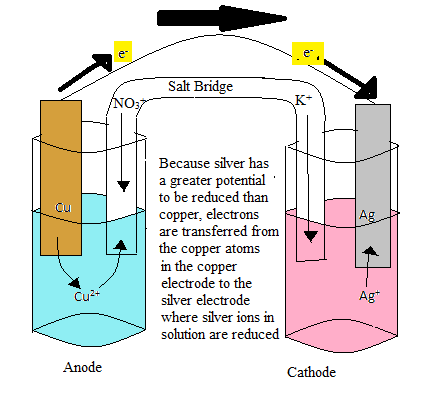
The batteries in your remote and the engine in your car are only a couple of examples of how chemical reactions create power through the flow of electrons. The cell potential is the way in which we can measure how much voltage exists between the two half cells of a battery. We will explain how this is done and what components allow us to find the voltage that exists in an electrochemical cell. IntroductionThe cell potential, Ecell, is the measure of the potential difference between two half cells in an electrochemical cell. The potential difference is caused by the ability of electrons to flow from one half cell to the other. Electrons are able to move between electrodes because the chemical reaction is a redox reaction. A redox reaction occurs when a certain substance is oxidized, while another is reduced. During oxidation, the substance loses one or more electrons, and thus becomes positively charged. Conversely, during reduction, the substance gains electrons and becomes negatively charged. This relates to the measurement of the cell potential because the difference between the potential for the reducing agent to become oxidized and the oxidizing agent to become reduced will determine the cell potential. The cell potential (Ecell) is measured in voltage (V), which allows us to give a certain value to the cell potential. Electrochemical CellAn electrochemical cell is comprised of two half cells. In one half cell, the oxidation of a metal electrode occurs, and in the other half cell, the reduction of metal ions in solution occurs. The half cell essentially consists of a metal electrode of a certain metal submerged in an aqueous solution of the same metal ions. The electrode is connected to the other half cell, which contains an electrode of some metal submerged in an aqueous solution of subsequent metal ions. The first half cell, in this case, will be marked as the anode. In this half cell, the metal in atoms in the electrode become oxidized and join the other metal ions in the aqueous solution. An example of this would be a copper electrode, in which the Cu atoms in the electrode loses two electrons and becomes Cu2+ .
The Cu2+ions would then join the aqueous solution that already has a certain molarity of Cu2+ ions. The electrons lost by the Cu atoms in the electrode are then transferred to the second half cell, which will be the cathode. In this example, we will assume that the second half cell consists of a silver electrode in an aqueous solution of silver ions. As the electrons are passed to the Ag electrode, the Ag+ ions in solution will become reduced and become an Ag atom on the Ag electrode. In order to balance the charge on both sides of the cell, the half cells are connected by a salt bridge. As the anode half cell becomes overwhelmed with Cu2+ ions, the negative anion of the salt will enter the solution and stabilized the charge. Similarly, in the cathode half cell, as the solution becomes more negatively charged, cations from the salt bridge will stabilize the charge. How does this relate to the cell potential?For electrons to be transferred from the anode to the cathode, there must be some sort of energy potential that makes this phenomenon favorable. The potential energy that drives the redox reactions involved in electrochemical cells is the potential for the anode to become oxidized and the potential for the cathode to become reduced. The electrons involved in these cells will fall from the anode, which has a higher potential to become oxidized to the cathode, which has a lower potential to become oxidized. This is analogous to a rock falling from a cliff in which the rock will fall from a higher potential energy to a lower potential energy. The difference between the anode's potential to become reduced and the cathode's potential to become reduced is the cell potential. Electrochemical cellHere is the list of the all the components:
All of these components create the Electrochemical Cell. How to measure the cell potential?
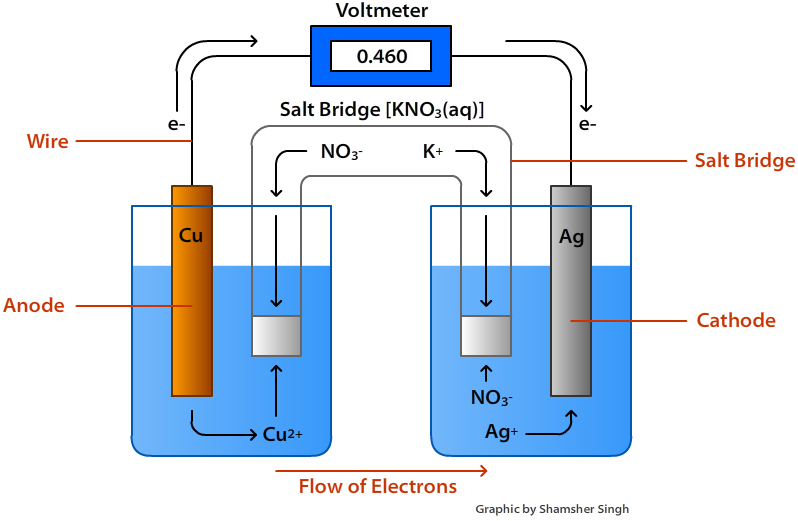
The image above is an electrochemical cell. The voltmeter at the very top in the gold color is what measures the cell voltage, or the amount of energy being produced by the electrodes. This reading from the voltmeter is called the voltage of the electrochemical cell. This can also be called the potential difference between the half cells, Ecell. Volts are the amount of energy for each electrical charge; 1V=1J/C: V= voltage, J=joules, C=coulomb. The voltage is basically what propels the electrons to move. If there is a high voltage, that means there is high movement of electrons. The voltmeter reads the transfer of electrons from the anode to the cathode in Joules per Coulomb. Cell Diagram
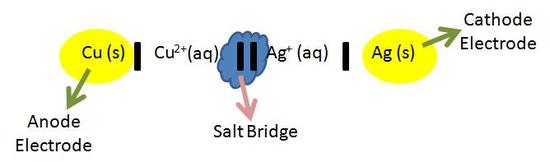
The image above is called the cell diagram. The cell diagram is a representation of the overall reaction in the electrochemical cell. The chemicals involved are what are actually reacting during the reduction and oxidation reactions. (The spectator ions are left out). In the cell diagram, the anode half cell is always written on the left side of the diagram, and in the cathode half cell is always written on the right side of the diagram. Both the anode and cathode are seperated by two vertical lines (ll) as seen in the blue cloud above. The electrodes (yellow circles) of both the anode and cathode solutions are seperated by a single vertical line (l). When there are more chemicals involved in the aqueous solution, they are added to the diagram by adding a comma and then the chemical. For example, in the image above, if copper wasn't being oxidized alone, and another chemical like K was involved, you would denote it as (Cu, K) in the diagram. The cell diagram makes it easier to see what is being oxidized and what is being reduced. These are the reactions that create the cell potential. Standard Cell PotentialThe standard cell potential (Eocell) is the difference of the two electrodes, which forms the voltage of that cell. To find the difference of the two half cells, the following equation is used: EoCell=EoCathode?EoAnode(1)with
The units of the potentials are typically measured in volts (V). Standard Cell Potential ExampleThe example will be using the picture of the Copper and Silver cell diagram. The oxadition half cell of the redox equation is: Cu(s) ? Cu2+(aq) + 2e- Eo= -0.340 V The reduction half cell of the redox equation is: ( Ag+ + e- ? Ag(s) ) x2 Eo= 0.800 V Complete redox equation: Cu(s) + 2Ag+ + 2e- ? Cu2+(aq) + 2Ag(s) + 2e- Simplified: Cu(s) + 2Ag+ ? Cu2+(aq) + 2Ag(s) Calculations: EoCell= Eo(Cathode) - Eo(Anode) EoCell = 0.800 V -- (0.340 V) EoCell= 0.800 V + (0.340 V) EoCell = 1.140 V The standard cell potential of this cell is 0.460 V. Important Standard Electrode (Reduction) PotentialsThe table below is a list of important standard electrode potentials in the reduction state. To determine oxidation electrodes, the reduction equation can simply be flipped and its potential changed from positive to negative (and vice versa). When using the half cells below, instead of changing the potential the equation below can be used without changing any of the potentials from positive to negative (and vice versa): EoCell= Eo(Cathode) + Eo(Anode) Table:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 >>
>>