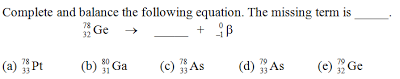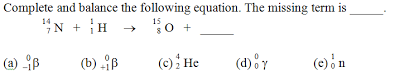Tags | |
UUID | 1a09bfc1-f145-11e9-8682-bc764e2038f2 |
End of Chapter 22 Problems
|
1.Which one of the following statements about nuclear reactions does not correctly distinguish nuclear reactions from ordinary chemical reactions? (a) Particles within the nucleus are involved. (b) No new elements can be produced. (c) Rate of reaction is independent of the presence of a catalyst. (d) Rate of reaction is independent of temperature. (e) They are often accompanied by the release of enormous amounts of energy. 2. Consider the following statements about the nucleus. Which of these statements is false? (a) Neutrons and protons together constitute the nucleus. (b) The nucleus is a sizable fraction of the total volume of the atom. (c) Electrons occupy essentially empty space around the nucleus. (d) Nearly all the mass of an atom resides in the nucleus. (e) The nuclei of all elements have approximately the same density. 3. A positron has a mass number of _____, a charge of _____, and a mass equal to that of a(an) _____. (a) 0, 1+, proton (b) 1, 2+, proton (c) 0, 1+, electron (d) 1, 2+, electron (e) 0, 0, proton 4. Which of the following statements about gamma rays is false? (a) Gamma rays have great penetrating power (b) They travel at the speed of light. (c) They are a common type of radiation emitted in decay processes. (d) They can be stopped by a thin layer of concrete or lead. (e) They have no mass. 5. Which of the following does not apply to a balanced nuclear reaction? (a) charge balance (b) mass balance (c) total number of nucleons remains the same (d) protons can turn into neutrons, or vice-versa (e) same number and kind of atoms on both sides of the equation |
Subpages (1): Answers to Problems
This Collection is empty
Collections
- Comments
- Attachments
- Stats
No comments |