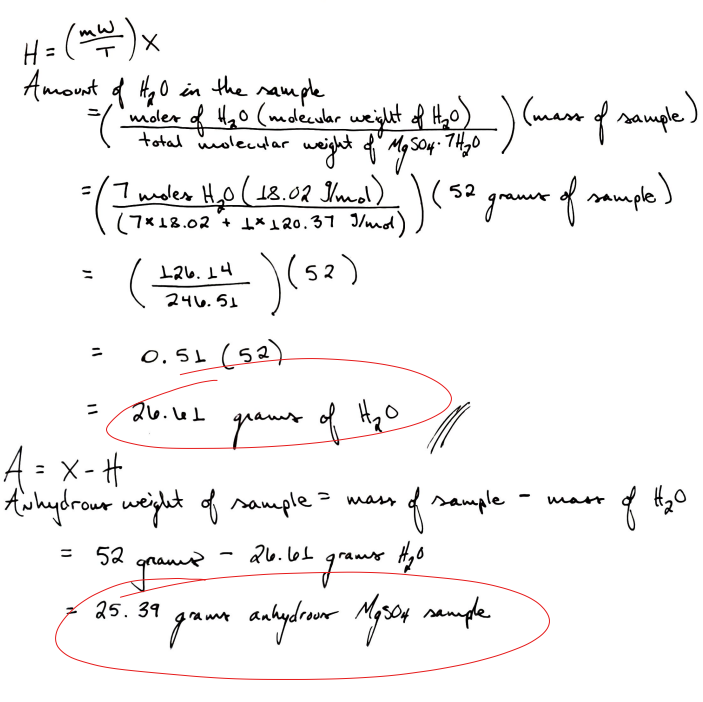The Amount of Water and Anhydrous Compound Calculator uses the mass of the sample, moles of water, moles of anhydrous compound, and the molecular weights of water and the compound to find the theoretical weight of the anhydrous compound and water.
Calculate the Amount of Water and Anhydrous Compound
- Enter the mass of the sample
- Enter the moles of H2O
- Enter the moles of the compound
- Enter the molecular weight of the compound
- If you need help calculating the molecular weight, use this calculator for compounds that have up to nine types of elements.
The Math / Science
Hydrous compounds are compounds that contain water with a definite mass in the form of H2O. When compounds undergo heat or vacuum processes with the intent of removing the water, they become anhydrous.
The Amount of Water and Anhydrous Compound Calculator uses the formula of the hydrous compound (i.e. CuCl2 ⋅ 2H2O) and the molecular weights of the compounds to find the theoretical amount of anhydrous compound and water within a sample. While this can also be found experimentally, it is useful to complete the theoretical math to check the success of the dehydrating processes used.
To find the amount of water (H2O), use the formula:
' H = (mW / T) X '
where:
H = the mass of water in the sample
m = number of moles of water
W = the mass of water in one mole of the compound
T = the molecular mass of the total hydrous compound (including mole count for water and compound)
X = the given or measured mass of the actual sample
To find the anhydrous weight, use the formula:
' A = X - H '
where:
A = the anhydrous weight of the compound or sample
X = the given or measured mass of the sample
H = the amount of water in the sample
Example
You have a 52 gram sample of magnesium sulfate hydrate (MgSO4 ⋅ 7H2O). This means that for every one mole of MgSO4 there are seven moles of pure water.
To find the mass of water and the magnesium sulfate individually, follow the picture below: