Tags | |
UUID | 19bf5376-f145-11e9-8682-bc764e2038f2 |
Catalysts
From UCDavis Chemwiki
Catalysts are substances that speed up a reaction but which are not consumed by it and do not appear in the net reaction equation. Also — and this is very important — catalysts affect the forward and reverse rates equally; this means that catalysts have no effect on the equilibrium constant and thus on the composition of the equilibrium state.
Catalysts provide alternative reaction pathways
Catalysts function by allowing the reaction to take place through an alternative mechanism that requires a smaller activation energy. This change is brought about by a specific interaction between the catalyst and the reaction components. You will recall that the rate constant of a reaction is an exponential function of the activation energy, so even a modest reduction of Ea can yield an impressive increase in the rate.
Catalysts are conventionally divided into two categories: homogeneous and heterogeneous. Enzymes, natural biological catalysts, are often included in the former group, but because they share some properties of both but exhibit some very special properties of their own, we will treat them here as a third category.
Some common examples which you may need for other parts of your syllabus include:
| reaction | catalyst |
|---|---|
| Decomposition of hydrogen peroxide | manganese(IV) oxide, MnO2 |
| Nitration of benzene | concentrated sulphuric acid |
| Manufacture of ammonia by the Haber Process | iron |
| Conversion of SO2 into SO3 during the Contact Process to make sulphuric acid | vanadium(V) oxide, V2O5 |
| Hydrogenation of a C=C double bond | nickel |
The importance of activation energy
Collisions only result in a reaction if the particles collide with a certain minimum energy called the activation energy for the reaction. You can mark the position of activation energy on a Maxwell-Boltzmann distribution to get a diagram like this:
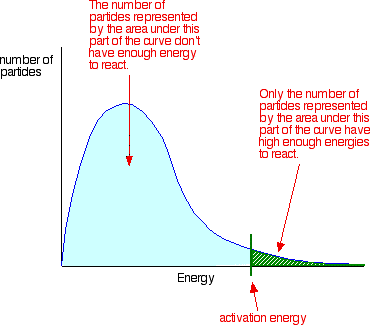
Only those particles represented by the area to the right of the activation energy will react when they collide. The great majority don't have enough energy, and will simply bounce apart.
To increase the rate of a reaction you need to increase the number of successful collisions. One possible way of doing this is to provide an alternative way for the reaction to happen which has a lower activation energy. In other words, to move the activation energy on the graph like this:
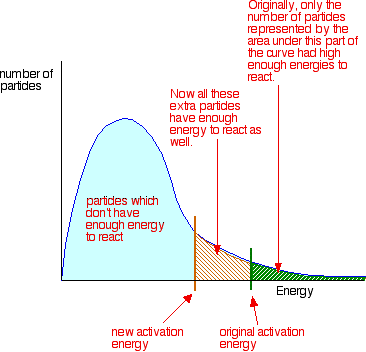
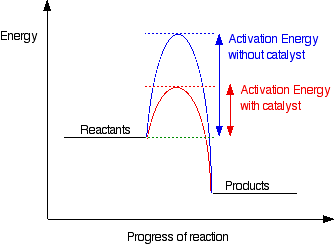
Adding a catalyst has exactly this effect on activation energy. A catalyst provides an alternative route for the reaction. That alternative route has a lower activation energy. Showing this on an energy profile:
A word of caution!
Be very careful when discussing how a catalyst operate. A catalyst provides an alternative route for the reaction with a lower activation energy. It does not "lower the activation energy of the reaction".
There is a subtle difference between the two statements that is easily illustrated with a simple analogy. Suppose you have a mountain between two valleys so that the only way for people to get from one valley to the other is over the mountain. Only the most active people will manage to get from one valley to the other.
Now suppose a tunnel is cut through the mountain. Many more people will now manage to get from one valley to the other by this easier route. You could say that the tunnel route has a lower activation energy than going over the mountain. But you haven't lowered the mountain! The tunnel has provided an alternative route but hasn't lowered the original one. The original mountain is still there, and some people will still choose to climb it.
In the chemistry case, if particles collide with enough energy they can still react in exactly the same way as if the catalyst wasn't there. It is simply that the majority of particles will react via the easier catalyzed route.
Homogeneous catalysis
As the name implies, homogeneous catalysts are present in the same phase (gas or liquid solution) as the reactants. Homogeneous catalysts generally enter directly into the chemical reaction (by forming a new compound or complex with a reactant), but are released in their initial form after the reaction is complete, so that they do not appear in the net reaction equation. Homogeneous catalysts react with one of the reactant molecules to form a more stable activated complex with a lower activation energy.
Heterogeneous catalysts
As its name implies, a heterogeneous catalyst exists as a separate phase (almost always a solid) from the one (most commonly a gas) in which the reaction takes place. The catalytic affect arises from disruption (often leading to dissociation) of the reactant molecules brought about by their interaction with the surface of the catalyst.Heterogeneous catalysts hold one reactant molecule in proper orientation for reaction to occur when the collision takes place.
Example 6
What effect does a catalyst have on the activation energy of a reaction? What effect does it have on the frequency factor (A)? What effect does it have on the change in potential energy for the reaction?
Subpages (1): Example 6
This Collection is empty
- Comments
- Attachments
- Stats
No comments |
