Tags | |
UUID | 19c7b7ff-f145-11e9-8682-bc764e2038f2 |
Reaction Mechanisms
From UCDavis Chemwiki
In chemistry, we sometimes find that looking at an overall reaction alone fails to tell us accurate information about the dynamics, and in particular the kinetics, of a reaction. Thus, we often need tools that allow us to understand a reaction in greater depth than just its reactants and products. Reaction Mechanisms act as tools to do this by allowing us to split an overall reaction into a series of intermediate reactions. These intermediate properties can then be examined individually and can collectively tell us much about the properties of the overall reactions that we see. As a result, reaction mechanisms have become an important field in chemistry and can especially tell us much about the kinetics of a reaction.
Introduction
Reaction mechanisms are step-by-step descriptions of what occurs molecularly in a given chemical reaction. Each step of the reaction mechanism is known as an elementary process, a term used to describe a moment in the reaction where one or more molecules changes geometry or is perturbed by the addition or omission of another interacting molecule. So collectively, an overall reaction and a reaction mechanism, is usually made up of multiple elementary processes. These elementary steps are the basic building blocks of a complex reaction and cannot be broken down any further.
Why are reaction mechanisms important?
- Reaction Mechanisms allow us to look beyond an often deceiving overall reaction.
- Most Importantly, reaction mechanisms allow us to estimate the rate law of an overall reaction. This topic is discussed below.
Situation 1
Take the reaction CO(g)+NO2(g)→ CO2(g)+NO(g). A look at this overall reaction would suggest that the carbon monoxide directly reacts with Nitrogen Dioxide to form the products. However, its reaction mechanism shows this is not the case. A look at two elementary processes show:
1) 2NO2(g)→ NO3(g)+NO(g)
2) CO(g)+NO3(g)→ CO2(g)+NO2(g)
Overall Net) CO(g)+NO2(g)→ CO2(g)+NO(g)
This reaction mechanism tells us that the compound NO_3 played a necessary role in allowing the reaction to take place, a concept not possible to know by looking at the overall equation.
Situation 2
Take the reaction 2NO(g)+O2(g)→2NO2(g). A look at this overall reaction might suggest that two NO molecules must collide with an oxygen to generate the so a product ensues. However, reactions with three molecules colliding at the same time, or termolecular processes, are extremely rare (this reaction is not rare) and so we must look to its reaction mechanism. A look at two elementary reactions show:
2NO(g)?N2O2(g) (1)
2N2O2(g)+O2(g)→2NO2(g) (2)
2NO(g)+O2(g)→2NO2(g) (overall reaction)
The reaction mechanism shows us that in fact, three molecules combining simultaneously to form a product does not happen. Rather, the overall reaction is a compilation of two individual elementary processes that take place.
Situation 3
Consider the chlorination of methane: CH4 (g)+Cl2 (g)→ CH3Cl(g)+HCl(g)
slow step: CH4 (g)+Cl2 (g)→ CH3 (g)+HCl(g) k1=[CH4 ][Cl2]
fast step: CH3 (g)+Cl2 (g)→ CH3 Cl(g)+Cl- (g) k2=[CH4 ][Cl2]
overall : CH4 (g)+2Cl2(g) → CH3Cl(g)+HCl(g)+Cl- (g)
Description of a Reaction Mechanism
Because a reaction mechanism is used to describe what occurs at each step of a reaction, it also describes the transition state, or the state when the maximum of potential energy is reached, in a reaction. A mechanism must show the order that the bonds form or break and the rate of each elementary step. Also accounted for in a mechanism are the reaction intermediates, which are stable molecules that do not appear in the experimentally determined rate law because they are formed in one step and consumed in a subsequent step. Since a reaction cannot proceed faster than the rate of slowest elementary step, the slowest step in a mechanism establishes the rate of the overall reaction. This elementary step is known as the rate-determining step.
A mechanism must satisfy the following two requirements:
- The elementary steps must add up to give the overall balanced equation for the reaction.
- The rate law for the rate-determining step must agree with the experimentally determined rate law.
Elementary Processes
Elementary processes are almost always either unimolecular or bimolecular. A unimolecular elementary process is when a single molecule dissociates. A bimolecular elementary process is when two molecules collide with each other to form products. A third process, called termolecular, is rare because it involves three molecules colliding at the same time. In the bimolecular and termolecular processes, the molecules may be different or the same.
Although a rate law for an overall reaction can only be experimentally determined, the rate law for each elementary step can be deduced from the chemical equation through inspection. A unimolecular elementary step has a first order rate law, whereas a bimolecular elementary step has a second order rate law.
The table below summarizes the types of elementary steps and the rate laws that they follow. A, B, and C here represent the reactants or reaction intermediates.
Elementary Steps and Rate Laws
| Molecularity | Elementary Step | Rate Law for Elementary step |
|---|---|---|
| 1 | A→ products | rate=k[A] |
| 2 | A+A→ products | rate=k[A]2 |
| A+B→ products | rate = k[A][B]` | |
| 3 | A+A+A→ products | rate=k[A]3 |
| A+2B→ products | rate=k[A][B]2 | |
| A+B+C→ products | rate=k[A][B][C] |
Elementary processes are also reversible. Some may reach conditions of equilibrium where both the foward and reverse reactions are equal.
Mechanism Example with a Slow Step and a Fast Step
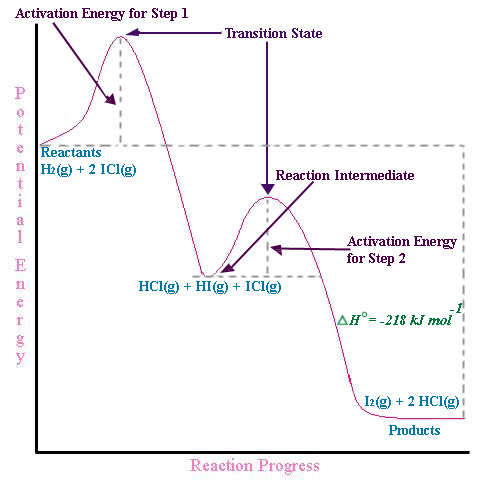
H2(g)+2ICl(g)→ I2(g)+2HCl(g)
The reaction between the gases iodine-monochloride and hydrogen produce two gases, iodine and hydrogen chloride as products. Through experiment, the rate law is found to be:
rate of reaction = k[H2][ICl]
Using a proposed reaction mechanism:
(1) slow reaction: H2+ICl→ HI+HCl Rate(1): k1 [H2][ICl]
(2) fast reaction: HI+ICl→ l2 +HCl Rate(2): k2 [HI][ICl]
Overall reaction: H2 +2ICl→ l2+2HCl
The mechanism chosen for the reaction above proposes that Step (1) is the rate-determining step, since it occurs slower. According to our mechanism, the rate law agrees that the mechanism works for this reaction. The rate of Step (1) = K1 [H2][ICl] is in agreement with the experimentally determined rate law, so the mechanism matches the stoichiometry of the overall reaction. However, this does not mean in any manner that the mechanism is proven to be correct. It only shows that the chosen mechanism works perfectly for this reaction. This is a reasonable proposal.
When there is enough energy for the slow reaction to react, the reaction moves to a faster step, Step (2). Step (2) occurs rapidly and our mechanism suggests that HI is consumed in the second step as fast as it is being formed. HI is, therefore, the reaction intermediate.
The diagram below shows the reaction progress of the two-step mechanism proposed. Notice how the reaction intermediate and the transition states do not last very long, but the intermediate can be isolated due to its fully formed bonds.
Diagram of a Two-Step Reaction
Example 5
In an alternative mechanism for the reaction of NO2 with CO, N2O4 appears as an intermediate.
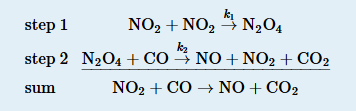
Write the rate law for each elementary reaction. Is this mechanism consistent with the experimentally determined rate law (rate = k[NO2]2)?
Subpages (1): Example 5
This Collection is empty
- Comments
- Attachments
- Stats
No comments |
