Tags | |
UUID | 19d3a33b-f145-11e9-8682-bc764e2038f2 |
Autoionization of Water
From UCDavis Chemwiki
As discussed earlier (in Part I of Chapter 10), water ionizes to hydrogen ions and hydroxide ions to an extremely small extent:
H2O(l) ⇌H+(aq)+OH-(aq) (1a)
Careful measurements show that at 25°C the concentrations of H+(aq) and OH–(aq) are each 1.005 × 107 mol/L. At higher temperatures more H+(aq) and OH–(aq) are produced while at lower temperatures less ionization of water occurs. Nevertheless, in pure water the concentration of H+(aq) always equals the concentration of OH–(aq). Dissolving acids or bases in water can change the concentrations of both H+(aq) and OH–(aq), causing them to differ from one another. The special case of a solution in which these two concentrations remain equal is called a neutral solution.A hydrogen ion, H+, is a hydrogen atom which has lost its single electron; that is, a hydrogen ion is just a proton. Because a proton is only about one ten-thousandth as big as an average atom or ion, water dipoles can approach very close to a hydrogen ion in solution. Consequently the proton can exert a very strong attractive force on a lone pair of electrons in a water molecule—strong enough to form a coordinate covalent bond:
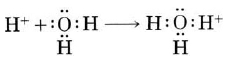
The H3O+ formed in this way is called a hydronium ion. All three of its O?H bonds are exactly the same, and the ion has a pyramidal structure as predicted by VSEPR theory. To emphasize the fact that a proton cannot exist by itself in aqueous solution, the chemical reaction is often rewritten a:
2H2O(l) ⇌ H3O+(aq)+OH-(aq)
Like other ions in aqueous solution, both hydronium and hydroxide ions are hydrated. Moreover, hydrogen bonds are involved in attracting water molecules to hydronium and hydroxide ions.
In both cases three water molecules appear to be rather tightly held, giving formulas H3O(H2O)3+ (or H9O4+) and HO(H2O)3– (or H7O4–). Possible structures for the hydrated hydronium and hydroxide ions are shown in Figure 1.
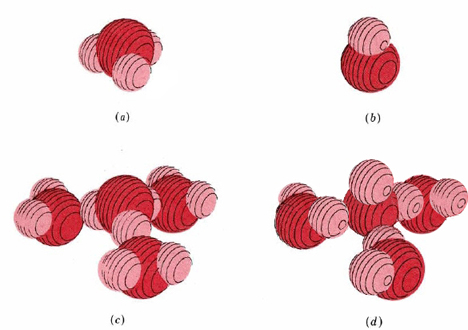
Figure 1 Hydrogen and hydroxide ions in aqueous solution. (a) Hydronium ion, H3O+; (b) hydroxide ion, OH–; (c) hydrated Hydronium ion, H9O4+; (d) hydrated hydroxide ion, H7O4–.
For a description of Figure 1, click the figure.Hydrogen bonding of hydronium and hydroxide ions to water molecules accounts rather nicely for the unusually large electrical currents observed for some electrolytes containing H and OH.
This Collection is empty
- Comments
- Attachments
- Stats
No comments |
