Chapter 14 Problems
1.Classify each of these as an Arrhenius acid or a base. Which are strong and which are weak? What ions are produced when each is dissolved in water?
a. `LiOH`
b. `Mg(OH)_2`
c. `HClO`
d. `HBr`
e. `KOH`
f. `H_2SO_3`
2. Why is HA considered a Bronsted-Lowery Acid?
3. In the equation depicting the autoionization of water,
`H_2O+H_2O -> H_3 O^(+) +OH^(-)`
would we say that the equilibrium lies to left, right, or neither?
4. Use chemical equations to illustrate the amphoteric properties of `HCO_3^(-)`.
5. What products will result when HSO4- is added to water? Complete and balance the reaction below.
`HSO_4^(-) + H_2O -> ?`
6. Identify the parent acid, parent base, conjugate acid and conjugate base for the following reaction:
-
- `HF+H_2O -> F^(-)+H_3O^(+)`
- `HSO_4^(-)+NH_3 -> SO_4^(-2)+NH^(+4)`
- `C_2H_3O_2^(-) +HCl -> HC_2H_3O_2+Cl^(-)`
- `HNO_2+H-2O-> H_3O^(+)+NO_2^(-)`
- `HCN+H_2O -> H-3O^(+) +CN^(-)`
7. Compare HCl with H2S in terms of acid strength.
- Compare H3PO4, NaH2PO4, Na2HPO4, and Na3PO4 with respect to acidity.
9. Identify the nature of each of the following as either a Lewis Acid or a Lewis Base:
a. `NH_3`
b. `Ag^(+)`
c. `Ni^(2+)`
d. `Pt^(4+)`
e. ` H_2 O`
f. `SO_2`
10. Explain why SiF4 can act as a Lewis Acid.
11. Identify each of the following as an acid or a base and write a chemical equation showing how it is an acid or base according to the Arrhenius definition:
a. `HNO_3 (aq)`
b. NH_4^(+) (aq)`
c. `KOH`
d. `HC_2 H_3 O_2 (aq)`
12. Identify each of the following as an acid or a base, then write the conjugate base of the acids and the conjugate acids of the bases.
a. `HCl`
b. `NH_3`
c. `H_2 SO_3
d. `ClO_4^(-)`
e. `HCHO_2`
f. `HF`
g. `CO_3^(2-)`
h. `HSO_4^(-)
13. Write chemical equations to show the amphoteric properties of H2PO4-
14. Order the following acids according to acid strength and explain how you determined this order:
`HClO`, `HClO_2`, `HClO_3`, `HClO_4`
15. Write the balanced equations for the following reactions between acid-base pairs.
a. `H_2CO_3 + Ba(OH)_2 ->`
b. `HBr + NaOH ->`
c. `H_2SO_4 + NH_3 ->`
d. `CH_3COOH + NaOH ->`
16. What are acidic salts?
17. Draw H2SO4 using a molecular modeling program and insert the electrostatic potential map. Take a screen capture of this molecule and identify the areas that cause sulfuric acid to be called a polyprotic acid. What does this term mean?
18. What is the relationship between the value of the equilibrium constant for the autoionization of liquid water and the tabulated value of the ion-product constant of liquid water (Kw)?
19. Show that water is amphiprotic by writing balanced chemical equations for the reactions of water with HNO3 and NH3. In which reaction does water act as the acid? In which does it act as the base?
20. The autoionization of sulfuric acid can be described by the following chemical equation:
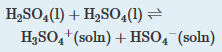
At 25°C, K = 3 × 10-4. Write an equilibrium constant expression for KH2SO4 that is analogous to Kw. The density of H2SO4 is 1.8 g/cm3at 25°C. What is the concentration of H3SO4+? What fraction of H2SO4 is ionized?
Subpages (1): Answers to EofCh 14 Problems
Equations
- Arrhenius Equation Juliet Use Equation
- Comments
- Attachments
- Stats
No comments |
