Hydronium and Hydroxide Ions
From UCDavis Chemwiki
If you refer to the following Table 1 Ions in Solution (Electrolytes) you will see that pure water does conduct some electrical current, albeit much less than-even the weak electrolytes listed there.
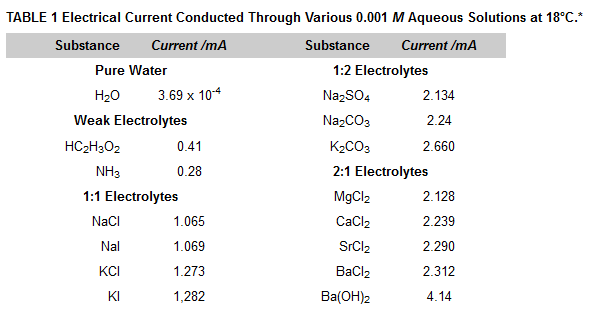
This is because water itself is a very weak electrolyte. It ionizes to hydrogen ions and hydroxide ions to an extremely small extent:
H2O(l) (1a)
A hydrogen ion, H+, is a hydrogen atom which has lost its single electron; that is, a hydrogen ion is just a proton. Because a proton is only about one ten-thousandth as big as an average atom or ion, water dipoles can approach very close to a hydrogen ion in solution. Consequently the proton can exert a very strong attractive force on a lone pair of electrons in a water molecule—strong enough to form a covalent bond:
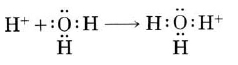
The H_3O^(+) formed in this way is called a hydronium ion. All three of its O-H bonds are exactly the same, and the ion has a pyramidal structure as predicted by VSEPR theory. To emphasize the fact that a proton cannot exist by itself in aqueous solution, this chemical reactions is often rewritten as:
2H_2O(l) ⇌ H_3O^(+)(aq) + OH^(-)(aq)