Lewis Theory
From UCDavis Chemwiki
Many oxyacids are rather unstable and cannot be isolated in pure form. An example is carbonic acid, H2CO3, which decomposes to water and carbon dioxide:
`H_2CO_3 (aq) ? H_2O(l) + CO_2(g)`
Since it can be made by removing H2O from H2CO3, CO2 is called the acid anhydride of H2CO3. (The term anhydride is derived from anhydrous, meaning “not containing water.”) Acid anhydrides are usually oxides of nonmetallic elements. Some common examples and their corresponding oxyacids are SO2—H2SO3; SO3—H2SO4; P4O10—H3PO4; N2O5—HNO3. Any of these anhydrides increases the hydronium-ion concentration when dissolved in water; for example,
`P4O_(10)(s) + 6H_2O(l) -> 4H_3 PO_4 (aq)`
`H_3 PO_4 (aq) + H_2 O(l) ? H_3 O+(aq) + H_2 PO_4^(-) (aq)`
In the Arrhenius sense, then, acid anhydrides are acids, but according to the Brönsted-Lowry definition, they are not acids because they contain no hydrogen.
In 1923, at the same time that the Brönsted-Lowry definition was proposed, G. N. Lewis suggested another definition which includes the acid anhydrides and a number of other substances as acids. According to the Lewis definition, an acid is any species which can accept a lone pair of electrons, and a base is any species which can donate a lone pair of electrons. An acid-base reaction in the Lewis sense involves formation of a coordinate covalent bond.
The Lewis definition has little effect on the types of molecules we expect to be basic. All the Brönsted-Lowry bases, for example, NH3, O2–, H–, contain at least one lone pair. Lewis’ idea does expand the number of acids, though. The proton (H+) is not the only species which can form a coordinate covalent bond with a lone pair. Cations of the transition metals, which are strongly hydrated, do the same thing:
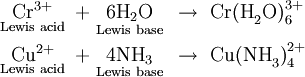
So can electron deficient compounds such as boron trifluoride:
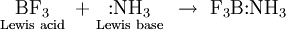
EXAMPLE 8 Identify the Lewis acids and bases in the following list. Write an equation for the combination of each acid with the Lewis base H2O.
(a) `BeCl_2(g)`
(b) `CH_3 OH`
(c) `SO_2`
(d) `CF_4`
This Collection is empty
- Comments
- Attachments
- Stats
No comments |
