Strong Acids and Bases
From UCDavis Chemwiki
The most straight-forward examples involving acids and bases deal with strong acids and bases. Strong acids, like HCl or HNO3, are such good proton donors that none of their own molecules can remain in aqueous solution, that is they nearly completely ionize (slightly <100%) in aqueous solution. All HCl molecules, for example, transfer their protons to H2O molecules, and so the solution contains only H3O+(aq) and Cl–(aq) ions. Similarly, the ions of strong bases, like LiOH or KOH, are such good proton acceptors that they cannot remain in aqueous solution, that is the nearly completely dissociate (slightly <100%) in aqueous solution.
Table 1 lists molecules and ions which act as strong acids and bases in aqueous solution. In addition to those which react completely with H2O to form H3O+ and OH–, any compound which itself contains these ions will serve as a strong acid or base. Note that the strength of an acid refers only to its ability to donate protons to H2O molecules and the strength of a base to its ability to accept protons from H2O molecules. The acidity or basicity of a solution, on the other hand, depends on the concentration as well as the strength of the dissolved acid or base.
TABLE 1 Species Which Are Strong Acids and Bases in Aqueous Solution.
| Strong Acids | Strong Bases |
| Binary Acids | |
| H3O+ HNO3 HCl HBr HI HClO4 (Acid strength increases as you move down the list). HNO3 and lower 100% ionized in dilute aq. soln. No molecules of nonionized acid. |
OH– NH2– (reacts completely with water to form OH–) LiOH NaOH KOH RbOH CsOH Ca(OH)2 Sr(OH)2 Ba(OH)2 (Strong bases are listed here in no specific order) |
| Ternary Acids | |
| H2SO4, HClO3 |
We observe that the hydronium ion is the strongest acid that can exist in aqueous solution. All acids stronger than H3O+(aq) react completely with water to produce H3O+(aq) and their conjugate bases. Similarly, the hyrdroxide ion is the strongest base that can exist in aqueous solution. Bases stronger than OH- react completely with water to produce OH- and their conjugate base.
Acid strength of ternary acids (containing three different elements) containing the same central element increase with increasing oxidation state of the central element and with increasing numbers of oxygen atoms.
H2SO3 <H2SO4
For most ternary acids containing different elements from the same periodic table group, in the same oxidation state, acid strengths increase with increasing electronegativity of the central element.
H2SeO4 <H2SO4
H3PO4 <HNO3
HBrO4<HClO4
At this point, we should also revisit the discussion about conjugate acid-base pairs we had previously and look at their relative strengths.
The use of conjugate acid-base pairs allows us to make a very simple statement about relative strengths of acids and bases. The stronger an acid, the weaker its conjugate base, and, conversely, the stronger a base, the weaker its conjugate acid. Take a moment to think about this fact and relate it to what we now know about strong acids/bases and their ionization/dissociation in aqueous solution.
TABLE 2 Some Important Conjugate Acid-Base Pairs Arranged in Order of Increasing Strength of the Base.
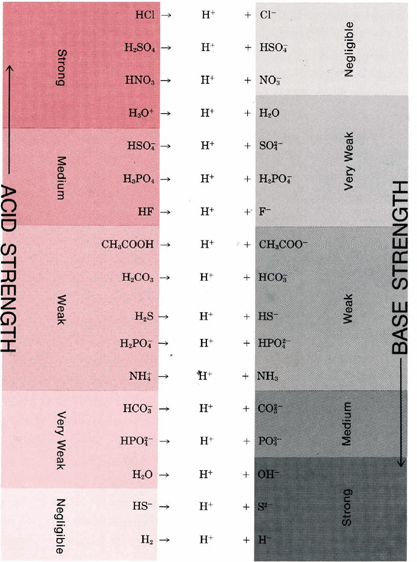
A strong acid like HCl donates its proton so readily that there is essentially no tendency for the conjugate base Cl– to reaccept a proton. Consequently, Cl– is a very weak base. A strong base like the H– ion accepts a proton and holds it so firmly that there is no tendency for the conjugate acid H2 to donate a proton. Hence, H2 is a very weak acid.
Table 2 gives a list of some of the more important conjugate acid-base pairs in order of increasing strength of the base. This table enables us to see how readily a given acid will react with a given base. The reactions with most tendency to occur are between the strong acids in the top left-hand comer of the table and the strong bases in the bottom right-hand comer. If a line is drawn from acid to base for such a reaction, it will have a downhill slope. By contrast, reactions with little or no tendency to occur (between the weak acids at the bottom left and the weak bases at the top right) correspond to a line from acid to base with an uphill slope. When the slope of the line is not far from horizontal, the conjugate pairs are not very different in strength, and the reaction goes only part way to completion. Thus, for example, if the acid HF is compared with the base CH3COO–, we expect the reaction to go part way to completion since the line is barely downhill.
As a general rule, strong proton donors are molecules in which a hydrogen is attached to a rather electronegative atom, such as oxygen or a halogen. Considerable electron density is shifted away from hydrogen in such a molecule, making it possible for hydrogen ions to depart without taking along any electrons. The strong acids in Table 1 fit this rule nicely. They are either hydrogen halides (HCl, HBr, HI) or oxyacids (whose general formula is HnXOm).
The strength of a base depends on its ability to attract and hold a proton. Therefore bases often have negative charges, and they invariably have at least one lone pair of electrons which can form a coordinate covalent bond to a proton. The strong bases in Table 1 might be thought of as being derived from neutral molecules by successive removal of protons. For example, OH– can be obtained by removing H+ from H2O. When the strong bases are considered this way, it is not surprising that they are good proton acceptors.
This Collection is empty
- Comments
- Attachments
- Stats
No comments |
