The Arrhenius Theory
From UCDavis Chemwiki
Acids
A typical example of an acid is hydrogen chloride gas, HCl(g). When it dissolves in water, HCl reacts to form hydronium ions and chloride ions:
HCl(g)+H2O(l)→ H3O+(aq)+Cl-(aq)
Thus the concentration of hydronium ions is increased above the value of 1.00 × 10–7 mol dm–3 characteristic of pure water (this fact will be discussed later in the semester). Other acids, such as nitric acid, HNO3 behave in the same way:
HNO3(l)+H2O(l)→ H3O+(aq)+NO-3(aq)
Thus the characteristic properties of solutions of acids are due to the presence of hydronium ions (or hydrogen ions). Whenever the concentration of hydronium ions exceeds 1.00 × 10–7 mol dm–3, an aqueous solution is said to beacidic. In 1884 a Swedish chemist, Svante Arrhenius (1859 to 1927), first recognized the importance of hydrogen ions. He defined an acid as any substance which increases the concentration of hydrogen (or hydronium) ions in aqueous solution.
The formation of a hydronium ion involves transfer of a proton from an acid molecule to a water molecule. This process is immediate?there are no free protons in solution which have left an acid molecule but have not yet attached themselves to a water molecule. To put it another way, a proton transfer is like a quarterback hand-off as opposed to a forward pass in foot- ball. The proton is always under the control and influence of one molecule or another. In the case of HCl we can indicate the transfer as

As the molecule collides with an H2O molecule, a hydrogen bond forms between the H and O atoms: Cl—H---OH2. When it begins to bounce away from the H2O molecule, the Cl atom loses control of the proton, leaving it attached to the O atom. The Cl atom retains control over both electrons which were in the H—Cl bond and thus ends up as a Cl–ion. The H2O molecule ends up with an extra proton, becoming H3O+.
Example 1.
Write balanced equations to describe the proton transfer which occurs when each of the following acids is dissolved in H2O:
(a) HClO4 (perchloric acid)
(b) HBr (hydrogen bromide, or hydrobromic acid).
Another point to note about proton transfers is that in any equation involving ions, the sum of the ionic charges on the left side must equal the sum of the ionic charges on the right. For example, the last overall equation in Example 1 has HBr and H2O on the left. Neither is an ion, and so the sum of the ionic charges is zero. On the right we have H3O+ and Br–, which satisfy the rule because +1 + (–1) + 0. An equation which does not satisfy this rule of charge balance will involve creation or destruction of one or more electrons and therefore cannot be valid. For example, the equation
2HBr→ 2H+ +Br2
cannot describe a valid proton transfer because the charges sum to zero on the left but +2 (because 2H+ ions) on the right. Careful examination reveals that there are 16 valence electrons (two octets in 2HBr) on the left but only 14 valence electrons (none in 2H+ and 14 in 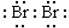 ) on the right. Two electrons have been destroyed—something which does not happen. Therefore the equation must be incorrect.
) on the right. Two electrons have been destroyed—something which does not happen. Therefore the equation must be incorrect.
Bases
The Arrhenius Theory also provided a definition for bases. According to this theory, a base is a substance that contains the OH (hydroxyl) group and produces hydroxide ions, OH-, in aqueous solution.
Where it falls short...
The Arrhenius theory of acids and bases satisfactorily explained reactions of protic acids with metal hydroxides. It was, however, limited in scope. For instance, the Arrhenius theory could not explain why NH3 displayed the properties of base. For that, we would need later theories of acids and bases, such as the Bronsted-Lowry Theory which will be discussed in Part 2 of this chapter.
Equations
- Arrhenius Equation Juliet Use Equation
- Comments
- Attachments
- Stats
No comments |
