Tags | |
UUID | 19de6907-f145-11e9-8682-bc764e2038f2 |
Titrations
From UCDavis Chemwiki
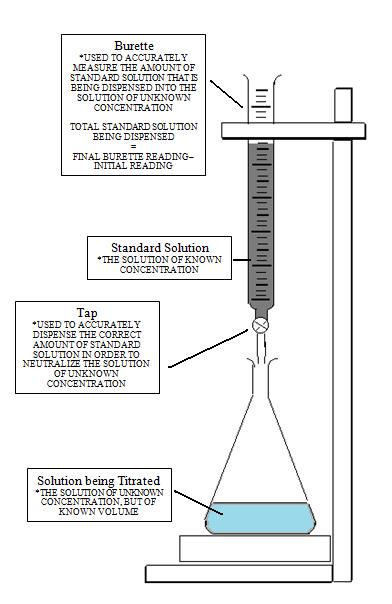
A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the "analyte") until the equivalence point is reached. The equivalence point is the point at which titrant has been added in exactly the right quantity to react stoichiometrically with the analyte. If either the titrant or analyte is colored, the equivalence point is evident from the disappearance of color as the reactants are consumed. Otherwise, an indicator may be added which has an "endpoint" (changes color) at the equivalence point, or the equivalence point may be determined from a titration curve. The amount of added titrant is determined from its concentration and volume:
and the amount of titrant can be used in the usual stoichiometric calculation to determine the amount of analyte.
Using "titration" as the keyword in YouTube finds many videos, including of an acid titrated with base and phenolphtalein indicator.
A measured volume of the solution to be titrated, in this case, colorless aqueous acetic acid, CH3COOH(aq) is placed in a beaker. The colorless sodium hydroxide NaOH(aq), which is the titrant, is added carefully by means of a buret. The volume of titrant added can then be determined by reading the level of liquid in the buret before and after titration. This reading can usually be estimated to the nearest hundredth of a milliliter, so precise additions of titrant can be made rapidly.
Figure 1

The initial reading of the buret. Placing a white card behind the buret can help the precision of the reading.As the first few milliliters of titrant flow into the flask, some indicator briefly changes to pink, but returns to colorless rapidly. This is due to a large excess of acetic acid. The limiting reagent NaOH is entirely consumed.
The added indicator changes to pink when the titration is complete, indicating that all of the aqueous acetic acid has been consumed by NaOH(aq). The reaction which occurs is
CH3COOH(aq)+NaOH(aq)→ Na+(aq)+CH3COO-(aq)+H2O(l) Eq (1)
Eventually, all the acetic acid is consumed. Addition of even a fraction of a drop of titrant produces a lasting pink color due to unreacted NaOH in the flask. The color change that occurs at the endpoint of the indicator signals that all the acetic acid has been consumed, so we have reached the equivalence point of the titration. If slightly more NaOH solution were added, there would be an excess and the color of the solution in the flask would get much darker. The endpoint appears suddenly, and care must be taken not to overshoot the endpoint.
For example, when a color indicator is being used:

To clear confusion, the endpoint and equivalence point are not necessarily equal, but they do represent the same idea. An endpoint is indicated by some form of indicator at the end of a titration. An equivalence point is when the moles of a standard solution (titrant) equal the moles of a solution of unknown concentration (analyte).
Indicators
The use of an indicator is key in performing a successful titration reaction. The purpose of the indicator is to show when enough standard solution has been added to fully react with the unknown concentration. However, an indicator should only be added when necessary and is dependent upon the solution that is being titrated. Therefore, indicators must only be added to the solution of unknown concentration when no visible reaction will occur. Depending on the solution being titrated, the choice of indicator can become key for the success of the titration.
After the titration has reached the endpoint, a final volume is read from the buret. Using the initial and final reading, the volume added can be determined quite precisely:
Figure 2

The final reading of the buret. If the liquid is colorless, placing a white card behind the buret can aid in precise readings.The object of a titration is always to add just the amount of titrant needed to consume exactly the amount of substance being titrated. In the NaOH—CH3COOH reaction [Eq. (1)], the equivalence point occurs when an equal molar amount of NaOH has been added from the graduated cylinder for every mole of CH3COOH originally in the titration flask. That is, at the equivalence point the ratio of the amount of NaOH, added to the amount of CH3COOH consumed must equal the stoichiometric ratio.
Solutions with accurately known concentrations are called standard solutions. Standard solutions are prepared through titration in a process known as standardization. In standardization we can determine the concentration of a solution be measuring accurately the volume of the solution required to react with an accurately known amount of aprimary standard (whose concentration is already known).
Figure 3
Types of Titrations
The following types of titrations are categorized based on chemical reactions.
Acid-Base Titration Reactions
Titration of acid/base reactions involve the process of neutralization in order to determine an unknown concentration. Acid-Base titrations can be made up of both strong and weak acids or bases. However, in order to determine the unknown concentration of an acid or base, you must add the opposite so that neutralization can be reached. Therefore, an acid of unknown concentration will be titrated using a basic standard solution and a base of unknown concentration will be titrated using an acidic standard solution. Examples of acid-base titrations include include:
- Titration of a Strong Acid with a Strong Base
- Titration of a Weak Acid with a Strong Base
- Titration of a Weak Base with a Strong Acid
- Titration of a Weak Polyprotic Acid
Acid-Base titrations often require the use of some kind of indicator depending on the strength of acid or base that is being titrated. In some cases a weak base or weak acid is used or a ph meter which reads the pH of the solution being titrated. Once the pH of the titrated solution equals seven, either indicated by a change in color or on a pH meter one can determine that titrations is complete.
Redox (Oxidizing-Reduction) Titrations
Another type of titration is the Redox, or Oxidizing-Reducing Titration, which is used to determine the oxidizing or reducing agent in a solution. When performing redox titrations, either the reducing or oxidizing agent will be used as the titrant against the other agent. The purpose of this titration is to determine the transfer of electrons from one substance to the other, similar to that of a redox reaction, in order to determine the reductant or oxidant. The end point of such titrations can be determined by either a color changing indicator or potentiometer.
Combination Reactions Titrations
Combination reaction titrations inclued two different types of titrations:
- One type of combination reaction titrations involve elements of opposite ions being titrated against each other. Therefore, one ion will act as the titrant while the other opposite ion will act as the analyte. However, combination reactions can involve more than two elements that are not necessarily ionic. These types of reactions will sometimes form a precipitate indicating the endpoint, but if not, some type of indicator may need to be added to the solution being titrated.
- Complex-formation Titrations: In these types of titrations the fomation of precipitate may or may not exist. Therefore, these types of titrations require the powerful complexing agent of ethlylenediaminetetraacetic acid (EDTA) or related compounds. For these type of reactions EDTA is used as a titrant becaue it will combine with many different types of cations in order to form a single type of complex. EDTA is most commonly used to determine the metal ions of a solution. However, EDTA should not be confused as being the indicator for these types of reactions, because the indicators are usually organic dyes. In fact EDTA merely acts as an inhibitor because it bonds strongly with the cations of metal, which results in the displacement of the indicator. This is what causes the color change, signifying the endpoint of titration.
Back Titrations
Back titrating should only be used when made necessary. The purpose of back titrating is to return to the endpoint after it was passed. It is often used when the solution being titrated is either too weak or too slow to give a reaction. It is also used if too much titrant was added, and the solution turned too dark. This means the experiment must be done over. The way to back titrate is to add an excess volume of another reactant of known concentration.
A back titration, or reverse titration, is most useful when the endpoint of a normal titration is too hard to identify.
This Collection is empty
- Comments
- Attachments
- Stats
No comments |
