Changes in Volume and Pressure
From UCDavis Chemwiki
This only applies to reactions involving gases.
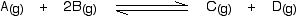
Increasing the pressure
According to Le Châtelier, the position of equilibrium will move in such a way as to counteract the change. That means that the position of equilibrium will move so that the pressure is reduced again. Pressure is caused by gas molecules hitting the sides of their container. The more molecules you have in the container, the higher the pressure will be. The system can reduce the pressure by reacting in such a way as to produce fewer molecules.
In this case, there are three molecules on the left-hand side of the equation, but only 2 on the right. By forming more C and D, the system causes the pressure to reduce. Increasing the pressure on a gas reaction shifts the position of equilibrium towards the side with fewer molecules.

N2 (g) + 3H2 (g) <-> 2NH3 (g)
If this mixture is transferred from a 1.5 L flask to a 5 L flask, in which direction does a net change occur to return to equilibrium?
Decreasing the pressure
The equilibrium will move in such a way that the pressure increases again. It can do that by producing more molecules. In this case, the position of equilibrium will move towards the left-hand side of the reaction.

What happens if there are the same number of molecules on both sides of the equilibrium reaction?
In this case, increasing the pressure has no effect whatsoever on the position of the equilibrium. Because you have the same numbers of molecules on both sides, the equilibrium cannot move in any way that will reduce the pressure again. Again, this isn't an explanation of why the position of equilibrium moves in the ways described.
Summary
Three ways to change the pressure of an equilibrium mixture are: 1. Add or remove a gaseous reactant or product, 2. Add an inert gas to the constant-volume reaction mixture or 3. Change the volume of the system. (2)
- Adding products makes Qc larger than Kc. This creates a net change in the reverse direction, toward reactants. The opposite occurs when adding more reactants. (2)
- Adding an inert gas into a gas-phase equilibrium at constant volume does not result in a shift. This is because the addition of a non-reactive gas does not change the partial pressures of the other gases in the container. While the total pressure of the system increases, the total pressure does not have any effect on the equilibrium constant. (1)
- When the volume of a mixture is reduced, a net change occurs in the direction that produces fewer moles of gas. When volume is increased the change occurs in the direction that produces more moles of gas.

This Collection is empty
- Comments
- Attachments
- Stats
No comments |
