Tags | |
UUID | 19e4ff3e-f145-11e9-8682-bc764e2038f2 |
Haber Process
From UCDavis Chemwiki
The Haber process, also known as the Haber-Bosch Process, was founded by Fritz Haber and Carl Bosch, both who were German Chemists. Haber discovered the conditions for the formation of ammonia, and Bosch discovered the work of high-pressure on chemical reactions (developed into industrial process). Both were awarded the Nobel Prize. During the 1920’s, there was a shortage of the world's supply for fixed nitrogen. Nitrogen was mainly used for fertilizer. Fertilizer was used in order to produce food, so that in WWI people could continue to fight. It only requires 1 percent of the world's energy to make 500 million tons of artificial fertilizer per year, which, in turn, helps feed 40 percent of the world's population.
THE PROCESS
The Haber process takes nitrogen gas from air and combines it with molecular hydrogen gas to form ammonia gas. This is an exothermic reaction, meaning it releases energy so that the sum of the enthalpies of N2 and H2 (the reactants) is greater than the enthalpy of NH3 (the products).
N2(g)+3H2(g)→ 2NH3(g) Δ
which is a reversible reaction:
2NH_3(g) -> N_2(g) + 3H_2(g) DeltaH=+ 92.4 kJ "mol"^(-1)
Here's a visual to help convey the process:
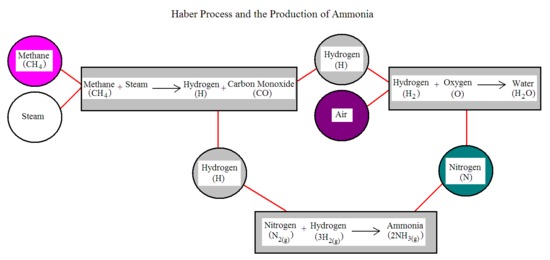
From the flow chart above, we can see that methane and steam combine to form hydrogen and carbon monoxide, which in turn releases hydrogen. The hydrogen then combines with oxygen from the air to produce water. Finally, nitrogen gas is released which combines with hydrogen gas to form ammonia. This takes place under high pressure and temperature and with an iron catalyst*** (mentioned later on).
Le Châtelier's Principle
The Haber process incorporates Le Chatlier's Principle, which is a good example of equilibrium principles. Uses of Le Chatlier's Principle are reversible reactions and reversible reactions involving gases. Chemical equilibrium is when a reaction has no tendency to change the quantity of the products and reactants, so the reaction can go both ways.
- Increasing the pressure and decreasing the temperature results in the higher yield of ammonia by causing a move of the reaction to the right.
- Because there are more molecules on the left side than the right side, when the pressure is increased, the system adapts to the change by moving the molecules left to right to decrease the overall pressure.
- For temperature, it moves from right to left when the temperature drops is because of the process being exothermic, where heat is released.
- The system adjusts to lessen the change, so it would make more heat to compensate for the energy lost, since that is the product of this. If more energy is made, then that would mean more ammonia is made, too. Even though decreasing the temperature is a slow reaction, if the temperature was increased to speed up the reaction, it would produce a smaller amount of ammonia yield.
This Collection is empty
- Comments
- Attachments
- Stats
No comments |
