Tags | |
UUID | 19e574fa-f145-11e9-8682-bc764e2038f2 |
Introduction
From UCDavis Chemiwiki
The concept that chemical processes may not evolve "to completion" but are content with stopping mid-way, results in a situation with partial reactants, products and potentially intermediates in a reaction. Identifying the relevant concentrations of these populations requires rigorous definition of new reaction parameters (e.g. "The Equilibration Constant").
Consider the following gas-phase chemical reaction: H2 +I2 →2HI
An apparatus contains 0.02 mole/L I2 gas in one syringe and 0.01 mole/L H2 gas in another syringe. When the two syringes are depressed, the two gases are mixed and the above reaction occurs.
As the reaction progresses, the concentrations of iodine and hydrogen decrease as they are consumed while the concentration of hydrogen iodide increases as it is formed. Eventually, however, the concentrations of all three species reach constant values. This behavior is a results of the back reaction in which hydrogen iodide reacts to form iodine and hydrogen. Initially there is no hydrogen iodide, so the back reaction cannot occur. As hydrogen iodide accumulates, the back reaction becomes significant.
Eventually the rate at which iodine is being consumed by the forward reaction is perfectly balanced by the rate at which it is being produced by the back reaction. The same is true for hydrogen and hydrogen iodide. When the rates of forward and reverse reactions are perfectly balanced in this way, the reaction is said to be at equilibrium.
A chemical reaction is in equilibrium when there is no tendency for the quantities of reactants and products to change. Chemical equilibria are dynamic equilibria; that is, individual molecules are continually reacting, even though the overall composition of the reaction mixture does not change.The direction in which we write a chemical reaction (and thus which components are considered reactants and which are products) is arbitrary. Thus the two equations
| H2 +I2 →2HI | "synthesis of hydrogen iodide" |
| 2HI→H2 +I2 | "dissociation of hydrogen iodide" |
represent the same chemical reaction system in which the roles of the components are reversed, and both yield the same mixture of components when the change is completed.
This last point is central to the concept of chemical equilibrium. It makes no difference whether we start with two moles of HI or one mole each of H2 and I2; once the reaction has run to completion, the quantities of these two components will be the same. In general, then, we can say that the composition of a chemical reaction system will tend to change in a direction that brings it closer to its equilibrium composition. Once this equilibrium composition has been attained, no further change in the quantities of the components will occur as long as the system remains undisturbed.
It's the same both ways
The two diagrams below show how the concentrations of the three components of this chemical reaction change with time. Examine the two sets of plots carefully, noting which substances have zero initial concentrations, and are thus "products" of the reaction equations shown. Satisfy yourself that these two sets represent the same chemical reaction system, but with the reactions occurring in opposite directions. Most importantly, note how the final (equilibrium) concentrations of the components are the same in the two cases.
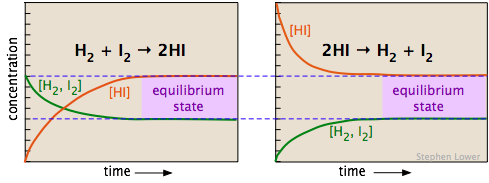
Whether we start with an equimolar mixture of H2 and I2 (left) or a pure sample of hydrogen iodide (shown on the right, using twice the initial concentration of HI to keep the number of atoms the same), the composition after equilibrium is attained (shaded regions on the right) will be the same.
The equilibrium composition is independent of the direction from which it is approached.
What is a reversible reaction?
A chemical equation of the form A→ B represents the transformation of A into B, but it does not imply that all of the reactants will be converted into products, or that the reverse reaction B→ A cannot also occur.
In general, both processes (forward and reverse) can be expected to occur, resulting in an equilibrium mixture containing finite amounts of all of the components of the reaction system. (We use the word components when we do not wish to distinguish between reactants and products.)
If the equilibrium state is one in which significant quantities of both reactants and products are present (as in the hydrogen iodide example given above), then the reaction is said to incomplete or reversible.
The latter term is preferable because it avoids confusion with "complete" in its other sense of being completed or finished, implying that the reaction has run its course and is now at equilibrium.
Note that there is no fundamental difference between the meanings of A→ B and A⇌B. Some older textbooks just use A=B.
- If it is desired to emphasize the reversibility of a reaction, the single arrow in the equation is replaced with a pair of hooked lines pointing in opposite directions, as in A⇌B.
- A reaction is said to be complete or quantitative when the equilibrium composition contains no significant amount of the reactants. However, a reaction that is complete when written in one direction is said "not to occur" when written in the reverse direction.
In principle, all chemical reactions are reversible, but this reversibility may not be observable if the fraction of products in the equilibrium mixture is very small, or if the reverse reaction is very slow (the chemist's term is "kinetically inhibited")
This Collection is empty
- Comments
- Attachments
- Stats
No comments |
