Tags | |
UUID | 19b9211a-f145-11e9-8682-bc764e2038f2 |
Chapter 12 Answers to Problems
1. ΔU=q+w
q = -3452 kJ (negative sign because the heat is released)
w = -11kJ (negative sign because work is being done by the system)
ΔU = -3452 kJ + (-11kJ) = -3463 kJ
2. First determine how many moles of acetone are present in the nail polish remover
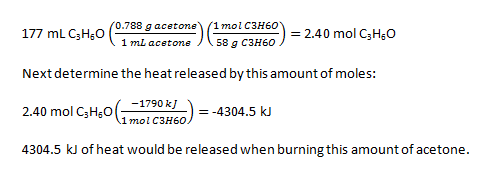
3.
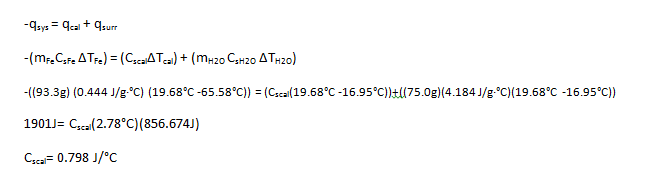 4.First we see that both equations are balanced. The enthalpies were given and there is no need to flip an equation around because it is possible to cancel out a couple terms as is. What is left is canceling out the O2 and the CO2species, writing the overall reaction and then summing the two enthalpies together.
4.First we see that both equations are balanced. The enthalpies were given and there is no need to flip an equation around because it is possible to cancel out a couple terms as is. What is left is canceling out the O2 and the CO2species, writing the overall reaction and then summing the two enthalpies together.
C(s, graphite) + O2 -> CO2
CO2 → C(s, diamond) + O2
Overall Equation becomes: C(s, graphite) → C(s, diamond)
- adding the enthalpies gives (-393.5 kJ/mol + 395.41 kJ/mol) = + 1.91 kJ/mol
Since the Ho is positive, the reaction is endothermic.
5. Given:
- mass of `C_(12) H(22) O_(12): 1.150 g
- Tinitial: 23.42°C
- Tfinal:27.64°C
- Heat of Capacity: 4.90 kJ/°C
SOLUTION
Using equation (2) calculate `q_"calorimeter":
qcalorimeter = (4.90 kJ/°C) x (27.64 - 23.42)°C = (4.90 x 4.22) kJ = 20.7 kJ
Plug into equation (1):
qrxn=Δqcalorimeter=-20.7kJ
But the question asks for kJ/mol C12H22O11, so this needs to be converted:
qrxn=-20.7kJ 1.150g C12H22O11=-18.0kJ C12H22O12
Per Mole C12H22O12:
qrxn=-18.0kJ
gC12H22O11 X 342.3g C12H22O111mol gC12H22O11 = -6.16 * 103kJ mol C12H22O11
6. a) 9/2 moles of gas reactants produce 5 moles of gas products. This increase in the number of moles of gas leads to an increase in entropy.
b) 3 moles of gas reactants produce only liquid product. This decrease in the number of gas moles leads to a decrease in entropy.
c) There are 6 moles of gas reactants and the same number of moles of gas products so we can not predict on this basis. One of the reactants is a solid. The total entropy of one mole of solid and six moles of gas (reactants) is less than the total enropy of six moles of gas and six moles of liquids (products) so we predict an increase in entropy.
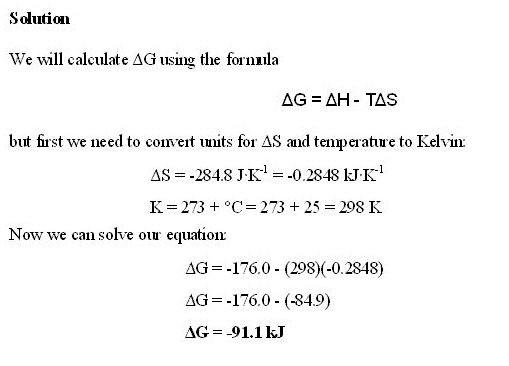
7. ΔGrxn = (1(-120.4 kJ/mol))-(1(0) +1(0))
ΔGrxn
8.
9.
This Collection is empty
- Comments
- Attachments
- Stats
No comments |
