Tags | |
UUID | 19bfcade-f145-11e9-8682-bc764e2038f2 |
Collision Theory of Reaction Rates
From ChemPRIME
Now that we know something about how reaction rates are defined, measured, and related to the concentrations of substances which participate in a reaction, we would like to be able to interpret these macroscopic observations in terms of some microscopic model. On the microscopic level, a chemical reaction involves transformation of reactant atoms, ions, and/or molecules into product atoms, ions, and/or molecules. This requires that some bonds be broken, other bonds be formed, and some nuclei be moved to new locations. There are a limited number of categories into which such microscopic transformations can be classified, and each of these can be related to a macroscopic rate law. Therefore studies of reaction rates provide some insight into what the atoms and molecules of reactants and products are doing as a reaction occurs.
Unimolecular
A reaction is said to be unimolecular if, on the microscopic level, rearrangement of the structure of a single molecule produces the appropriate product molecules. An example of a unimolecular process is conversion of cis-2-butene totrans-2-butene (in the absence of any catalyst):
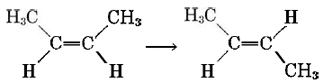
All that is required for this reaction to occur is a twist or rotation around the double bond, interchanging the methyl group with the hydrogen atom on the right-hand side. Only one cis-2-butene molecule need be involved as a reactant in this process.
Bimolecular
A second type of microscopic process which can result in a chemical reaction involves collision of two particles. Such a process is called a bimolecular process. A typical example of a bimolecular process is the reaction between nitrogen dioxide and carbon monoxide:
NO2 +CO→ NO+CO2 (1)
Here an O atom is transferred from NO2 to CO when the two molecules collide.
Several factors affect the rate of a bimolecular reaction. The first of these is the frequency of collisions between the two reactant molecules. Suppose we have a single molecule of type A (shown in black in Fig. 1a, or in blue in the animation) moving around in a gas which otherwise consists entirely of molecules of kind B (indicated in white in the figure, light blue in the animation). If we double the concentration of B molecules (Fig. 1b), the number of collisions during the same time period doubles, because there are now twice as many B molecules to get in the way. Similarly, if we put twice as many A molecules into the original container, each of them collides with B molecules the same number of times, again giving twice as many A-B collisions.
Termolecular
A termolecular process is one which involves simultaneous collision of three microscopic particles. In the gas phase, termolecular processes occur much less often than bimolecular processes, because the probability of three molecules all coming together at the same time is less than a thousandth the probability that two molecules will collide.
A+B+C→D
Consequently it is commonly found that if three molecules, A, B, and C, must combine during the course of a reaction, they will do so stepwise. That is, B and C might first combine in a bimolecular process to form BC, which would then combine with A in a second bimolecular process.
B+C→BC
BC+A→D
This can usually happen many times over before a successful termolecular collision would occur.
This Collection is empty
- Comments
- Attachments
- Stats
No comments |
