Tags | |
UUID | 19d41e01-f145-11e9-8682-bc764e2038f2 |
Conjugate Acid-Base Pairs
From UCDavis Chemwiki
The examples found in the previous sections on acids and bases proton-transfer processes can be broken into two hypothetical steps: (1) donation of a proton by an acid, and (2) acceptance of a proton by a base. (Water served as the base in the acid example and as the acid in the base example in the Bronsted-Lowry section of this chapter). The hypothetical steps are useful because they make it easy to see what species is left after an acid donated a proton and what species is formed when a base accepted a proton. We shall use hypothetical steps or half-equations in this section, but you should bear in mind that free protons never actually exist in aqueous solution.
Suppose we first consider a weak acid, the ammonium ion. When it donates a proton to any other species, we can write the half-equation
NH+4 → H+ +NH3
But NH3 is one of the compounds we know as a weak base. In other words, when it donates a proton, the weak acid NH4+ is transformed into a weak base NH3. Another example, this time starting with a weak base, is provided by fluoride ion:
F- +H+ → HF
The proton converts a weak base (F-) into a weak acid (HF).
The situation just described for NH4+ and NH3or for F– and HF applies to all acids and bases. The original acid or base (in this example NH4+ and F– ) are referred to as the parent or original acid or base. Whenever an acid donates a proton, the acid changes into a base, and whenever a base accepts a proton, an acid is formed. An acid and a base which differ only by the presence or absence of a proton are called a conjugate acid-base pair. Thus NH3 is called the conjugate base of NH4+, and NH4+ is the conjugate acid of NH3. Similarly, HF is the conjugate acid of F–, and F– the conjugate base of HF.
Example 3.
What is the conjugate acid or the conjugate base of
(a) HCl
(b) CH3NH2
(c) OH-
(d) HCO-3
TABLE 2 Some Important Conjugate Acid-Base Pairs Arranged in Order of Increasing Strength of the Base.
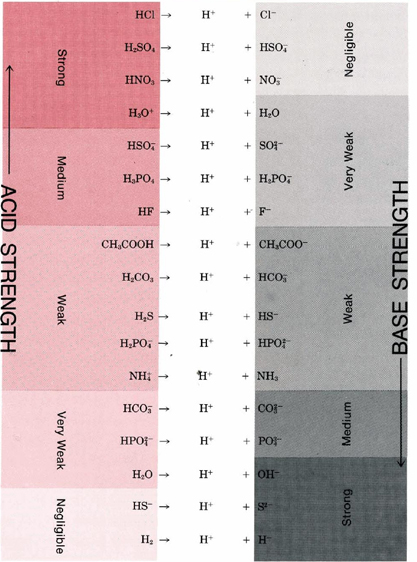
A strong acid like HCl donates its proton so readily that there is essentially no tendency for the conjugate base Cl– to reaccept a proton. Consequently, Cl– is a very weak base. A strong base like the H– ion accepts a proton and holds it so firmly that there is no tendency for the conjugate acid H2 to donate a proton. Hence, H2 is a very weak acid.
Table 2 gives a list of some of the more important conjugate acid-base pairs in order of increasing strength of the base. This table enables us to see how readily a given acid will react with a given base. The reactions with most tendency to occur are between the strong acids in the top left-hand comer of the table and the strong bases in the bottom right-hand comer. If a line is drawn from acid to base for such a reaction, it will have a downhill slope. By contrast, reactions with little or no tendency to occur (between the weak acids at the bottom left and the weak bases at the top right) correspond to a line from acid to base with an uphill slope. When the slope of the line is not far from horizontal, the conjugate pairs are not very different in strength, and the reaction goes only part way to completion. Thus, for example, if the acid HF is compared with the base CH3COO–, we expect the reaction to go part way to completion since the line is barely downhill.
Example 4.
Write a balanced equation to describe the reaction which occurs when a solution of potassium hydrogen sulfate, KHSO4, is mixed with a solution of sodium bicarbonate, NaHCO3. Identify the conjugate acid-base pairs by labeling the parent acid and base and the resulting conjugate acid and base.
Example 5.
What reactions will occur when an excess of acetic acid is added to a solution of potassium phosphate, K3PO4?
This Collection is empty
- Comments
- Attachments
- Stats
No comments |
