26.2 Quantization of charge by Benjamin Crowell, Light and Matter licensed under the Creative Commons Attribution-ShareAlike license.
26.2 Quantization of charge
Proving that atoms actually existed was a big accomplishment, but demonstrating their existence was different from understanding their properties. Note that the Brown-Einstein observations had nothing at all to do with electricity, and yet we know that matter is inherently electrical, and we have been successful in interpreting certain electrical phenomena in terms of mobile positively and negatively charged particles. Are these particles atoms? Parts of atoms? Particles that are entirely separate from atoms? It is perhaps premature to attempt to answer these questions without any conclusive evidence in favor of the charged-particle model of electricity.
 Strong support for the charged-particle model came from a 1911 experiment by physicist Robert Millikan at the University of Chicago. Consider a jet of droplets of perfume or some other liquid made by blowing it through a tiny pinhole. The droplets emerging from the pinhole must be smaller than the pinhole, and in fact most of them are even more microscopic than that, since the turbulent flow of air tends to break them up. Millikan reasoned that the droplets would acquire a little bit of electric charge as they rubbed against the channel through which they emerged, and if the charged-particle model of electricity was right, the charge might be split up among so many minuscule liquid drops that a single drop might have a total charge amounting to an excess of only a few charged particles --- perhaps an excess of one positive particle on a certain drop, or an excess of two negative ones on another.
Strong support for the charged-particle model came from a 1911 experiment by physicist Robert Millikan at the University of Chicago. Consider a jet of droplets of perfume or some other liquid made by blowing it through a tiny pinhole. The droplets emerging from the pinhole must be smaller than the pinhole, and in fact most of them are even more microscopic than that, since the turbulent flow of air tends to break them up. Millikan reasoned that the droplets would acquire a little bit of electric charge as they rubbed against the channel through which they emerged, and if the charged-particle model of electricity was right, the charge might be split up among so many minuscule liquid drops that a single drop might have a total charge amounting to an excess of only a few charged particles --- perhaps an excess of one positive particle on a certain drop, or an excess of two negative ones on another.
Millikan's ingenious apparatus, e, consisted of two metal plates, which could be electrically charged as needed. He sprayed a cloud of oil droplets into the space between the plates, and selected one drop through a microscope for study. First, with no charge on the plates, he would determine the drop's mass by letting it fall through the air and measuring its terminal velocity, i.e., the velocity at which the force of air friction canceled out the force of gravity. The force of air drag on a slowly moving sphere had already been found by experiment to be bvr2, where b was a constant. Setting the total force equal to zero when the drop is at terminal velocity gives
bvr2-mg=0,
and setting the known density of oil equal to the drop's mass divided by its volume gives a second equation,
ρ=m43πr3.
Everything in these equations can be measured directly except for m and r, so these are two equations in two unknowns, which can be solved in order to determine how big the drop is.
Next Millikan charged the metal plates, adjusting the amount of charge so as to exactly counteract gravity and levitate the drop. If, for instance, the drop being examined happened to have a total charge that was negative, then positive charge put on the top plate would attract it, pulling it up, and negative charge on the bottom plate would repel it, pushing it up. (Theoretically only one plate would be necessary, but in practice a two-plate arrangement like this gave electrical forces that were more uniform in strength throughout the space where the oil drops were.) When the drop was being levitated, the gravitational and electric forces canceled, so qE=mg. Since the mass of the drop, the gravitational field g, and the electric field E were all known, the charge q could be determined.
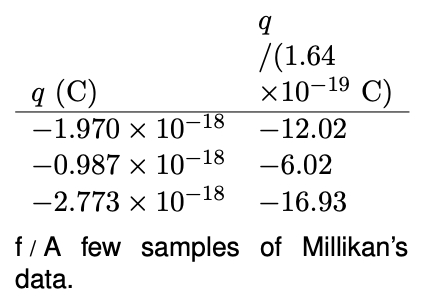 Table f shows a few of the results from Millikan's 1911 paper. (Millikan took data on both negatively and positively charged drops, but in his paper he gave only a sample of his data on negatively charged drops, so these numbers are all negative.) Even a quick look at the data leads to the suspicion that the charges are not simply a series of random numbers. For instance, the second charge is almost exactly equal to half the first one. Millikan explained the observed charges as all being integer multiples of a single number, 1.64×10-19C. In the second column, dividing by this constant gives numbers that are essentially integers, allowing for the random errors present in the experiment. Millikan states in his paper that these results were a
Table f shows a few of the results from Millikan's 1911 paper. (Millikan took data on both negatively and positively charged drops, but in his paper he gave only a sample of his data on negatively charged drops, so these numbers are all negative.) Even a quick look at the data leads to the suspicion that the charges are not simply a series of random numbers. For instance, the second charge is almost exactly equal to half the first one. Millikan explained the observed charges as all being integer multiples of a single number, 1.64×10-19C. In the second column, dividing by this constant gives numbers that are essentially integers, allowing for the random errors present in the experiment. Millikan states in his paper that these results were a
... direct and tangible demonstration ... of the correctness of the view advanced many years ago and supported by evidence from many sources that all electrical charges, however produced, are exact multiples of one definite, elementary electrical charge, or in other words, that an electrical charge instead of being spread uniformly over the charged surface has a definite granular structure, consisting, in fact, of ... specks, or atoms of electricity, all precisely alike, peppered over the surface of the charged body.
In other words, he had provided direct evidence for the charged-particle model of electricity and against models in which electricity was described as some sort of fluid. The basic charge is notated e, and the modern value is e=1.60×10-19C. The word “quantized” is used in physics to describe a quantity that can only have certain numerical values, and cannot have any of the values between those. In this language, we would say that Millikan discovered that charge is quantized. The charge e is referred to as the quantum of charge.
self-check:
Is money quantized? What is the quantum of money?
(answer in the back of the PDF version of the book)
A historical note on Millikan's fraud
Very few undergraduate physics textbooks mention the well-documented fact that although Millikan's conclusions were correct, he was guilty of scientific fraud. His technique was difficult and painstaking to perform, and his original notebooks, which have been preserved, show that the data were far less perfect than he claimed in his published scientific papers. In his publications, he stated categorically that every single oil drop observed had had a charge that was a multiple of e, with no Exceptions or omissions. But his notebooks are replete with notations such as “beautiful data, keep,” and “bad run, throw out.” Millikan, then, appears to have earned his Nobel Prize by advocating a correct position with dishonest descriptions of his data.
26.2 Quantization of charge by Benjamin Crowell, Light and Matter licensed under the Creative Commons Attribution-ShareAlike license.